

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50302828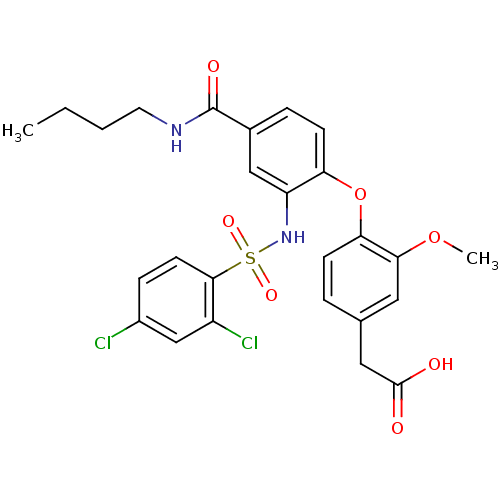 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PGD2-induced CRTH2 receptor internalization of CD16 negative granulocytes in human whole blood by flow cytometry | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50229378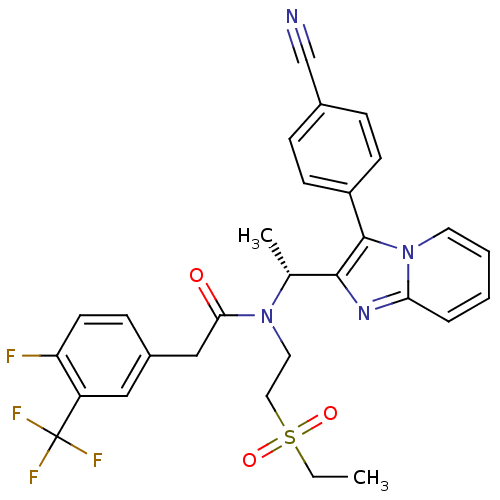 ((R)-N-(1-(3-(4-cyanophenyl)H-imidazo[1,2-a]pyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA | Bioorg Med Chem Lett 18: 688-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.060 BindingDB Entry DOI: 10.7270/Q2WQ03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50302828 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at DP receptor in human platelets assessed as inhibition of PGD2-induced cAMP production by competitive ELISA | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983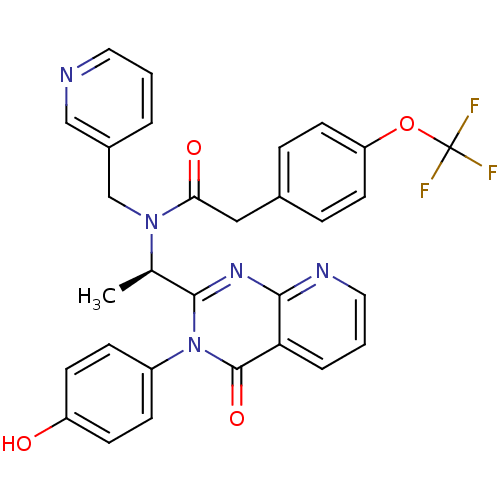 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate assessed as unbound inhibitor concentration required for half maximal enz... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate assessed as unbound inhibitor concentration required for half maximal ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate assessed as residual enzyme activity after 2 to 10 mins by LC-MS/MS an... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate assessed as residual enzyme activity after 2 to 10 mins by LC-MS/MS analy... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50302828 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of EP4 expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP production | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50302828 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of EP2 expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP production | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50458169 (Avagacestat | BMS 708163 | BMS-708163 | BMS-708163...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492082 (CHEMBL2396959) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492094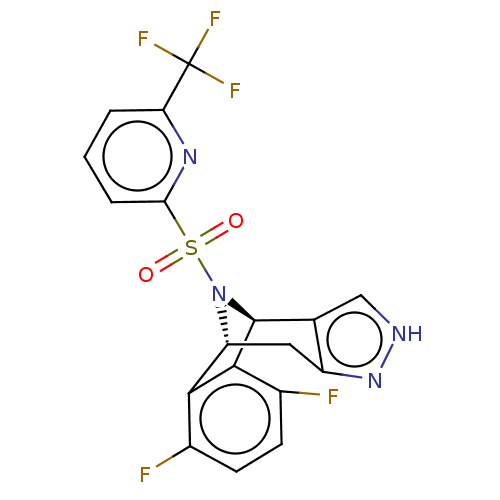 (CHEMBL2396964) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492093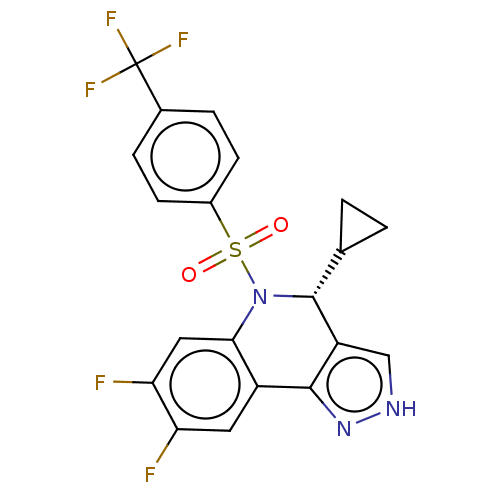 (CHEMBL2396778) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50310487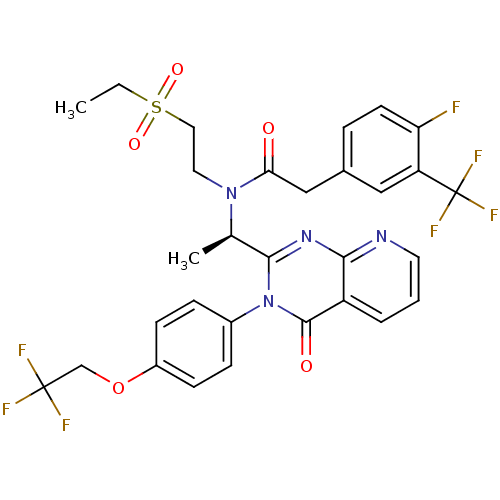 ((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity against human CXCR3 expressed in human PBMC assessed as inhibition of cell migration in response to IP10 in buffer | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50371494 (CHEMBL256589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from human recombinant CXCR3 receptor expressed in IL2-activated human PBMC | Bioorg Med Chem Lett 18: 608-13 (2008) Article DOI: 10.1016/j.bmcl.2007.11.072 BindingDB Entry DOI: 10.7270/Q2668F1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492085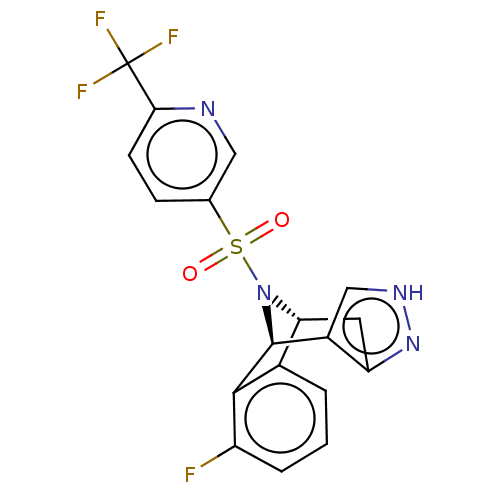 (CHEMBL2396953) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492083 (CHEMBL2396960) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50310487 ((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity against human CXCR3 expressed in human PBMC assessed as inhibition of cell migration in response to MIG in buffer | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50229439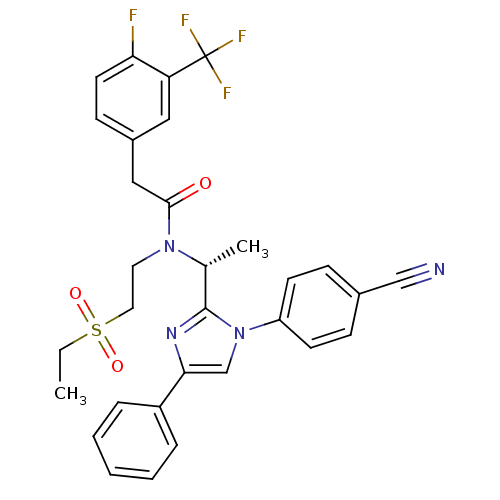 ((R)-N-(1-(1-(4-cyanophenyl)-4-phenyl-1H-imidazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from human recombinant CXCR3 receptor expressed in IL2-activated human PBMC | Bioorg Med Chem Lett 18: 608-13 (2008) Article DOI: 10.1016/j.bmcl.2007.11.072 BindingDB Entry DOI: 10.7270/Q2668F1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50229439 ((R)-N-(1-(1-(4-cyanophenyl)-4-phenyl-1H-imidazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50229381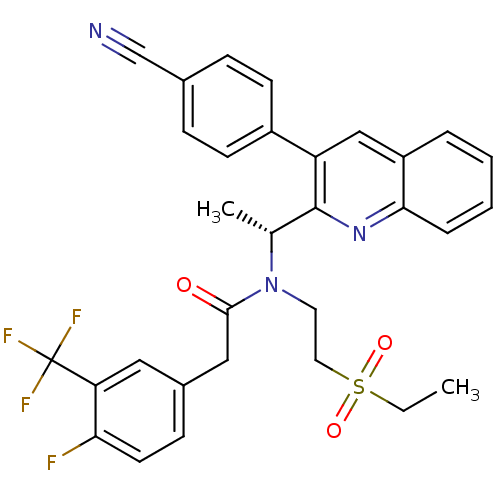 ((R)-N-(1-(3-(4-cyanophenyl)quinolin-2-yl)ethyl)-N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA | Bioorg Med Chem Lett 18: 688-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.060 BindingDB Entry DOI: 10.7270/Q2WQ03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50310487 ((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity against human CXCR3 expressed in human PBMC assessed as inhibition of cell migration in response to ITAC in RPMI buffer | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300900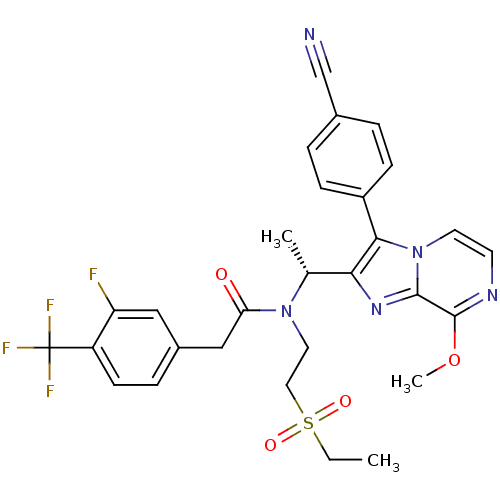 ((R)-N-(1-(3-(4-cyanophenyl)-8-methoxyimidazo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300899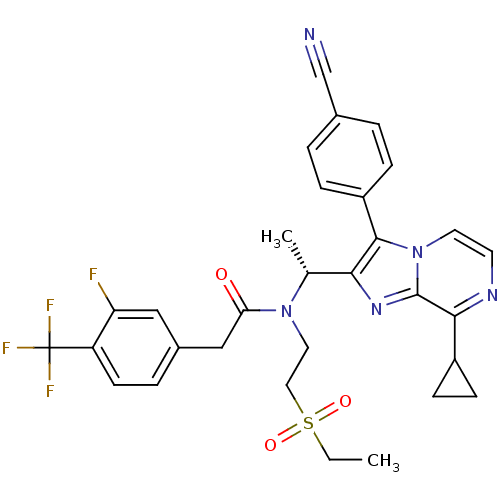 ((R)-N-(1-(3-(4-cyanophenyl)-8-cyclopropylimidazo[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300905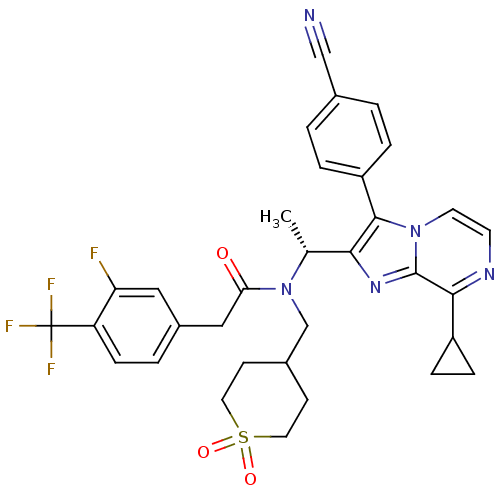 (CHEMBL578192 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300907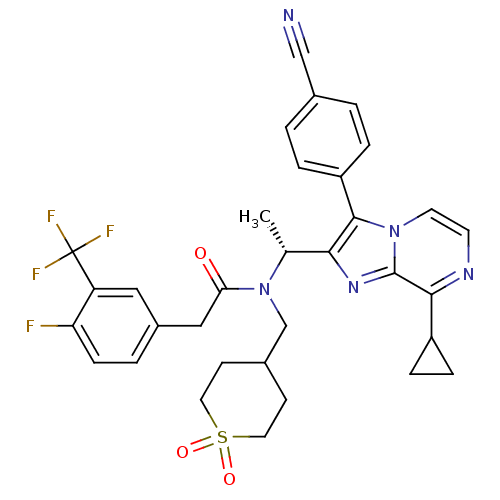 (CHEMBL576097 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50229462 ((R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydroquin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from human recombinant CXCR3 receptor expressed in IL2-activated human PBMC | Bioorg Med Chem Lett 18: 608-13 (2008) Article DOI: 10.1016/j.bmcl.2007.11.072 BindingDB Entry DOI: 10.7270/Q2668F1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50310487 ((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ITAC from human CXCR3 expressed in human PBMC after 2 hrs in RPMI buffer by scintillation counting | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50310487 ((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-1P10 from human CXCR3 expressed in PBMC after 2 hrs in RPMI buffer by scintillation counting | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352621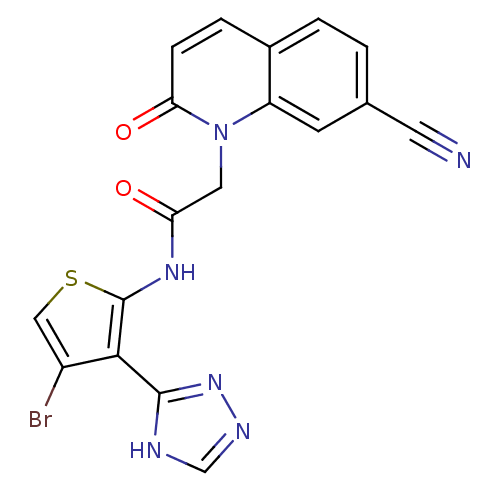 (CHEMBL1822152) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50310486 ((R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-1P10 from human CXCR3 expressed in PBMC after 2 hrs in RPMI buffer by scintillation counting | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352620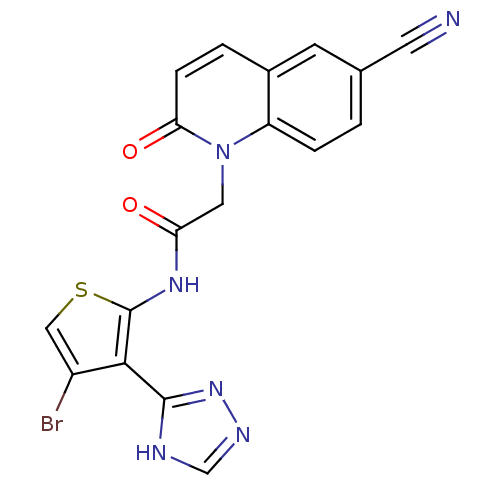 (CHEMBL1822151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492098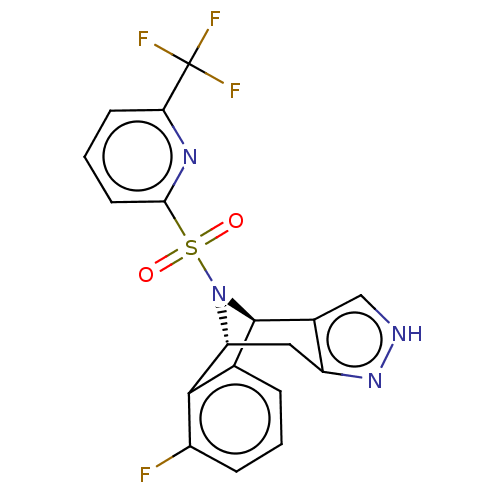 (CHEMBL2396963) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50310483 ((R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-1P10 from human CXCR3 expressed in PBMC after 2 hrs in RPMI buffer by scintillation counting | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50352624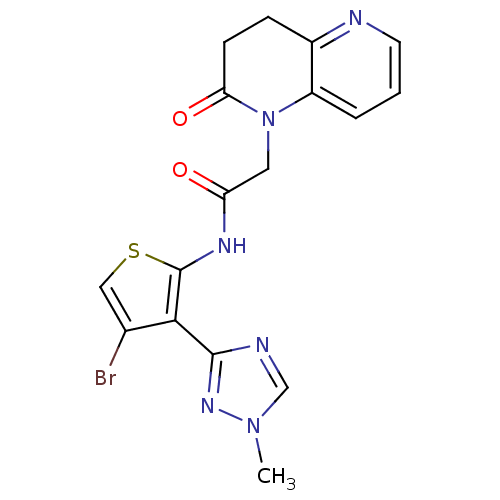 (CHEMBL1822305 | US9796706, Compound 139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300909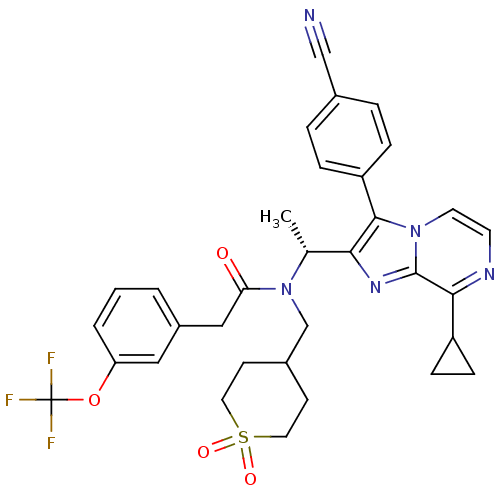 (CHEMBL576099 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300906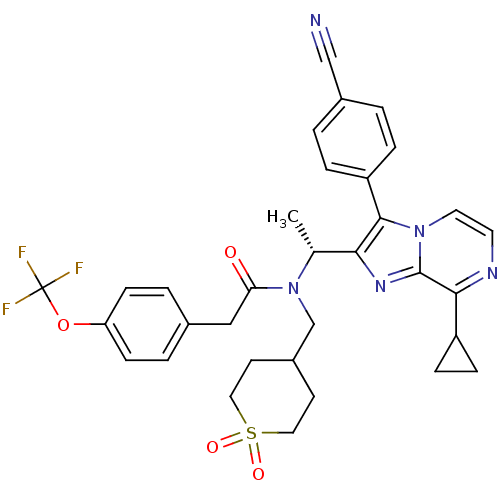 (CHEMBL576096 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300902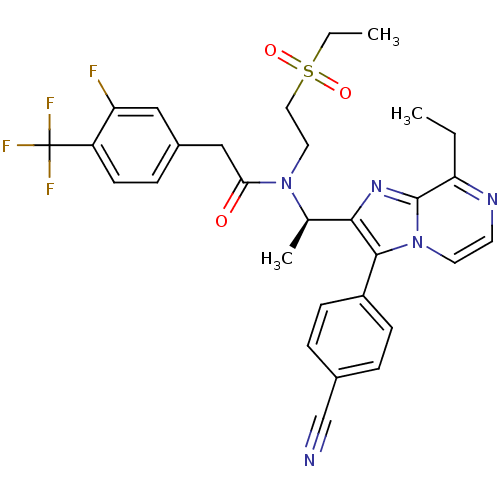 ((R)-N-(1-(3-(4-cyanophenyl)-8-ethylimidazo[1,2-a]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492097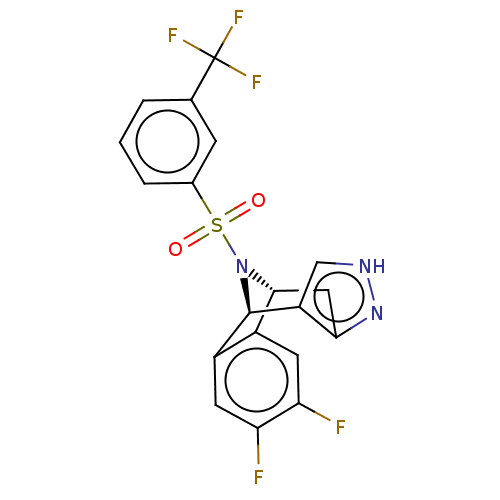 (CHEMBL2396965) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300898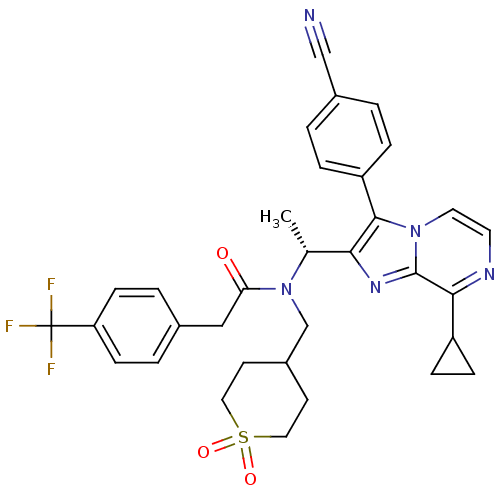 (CHEMBL570858 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300903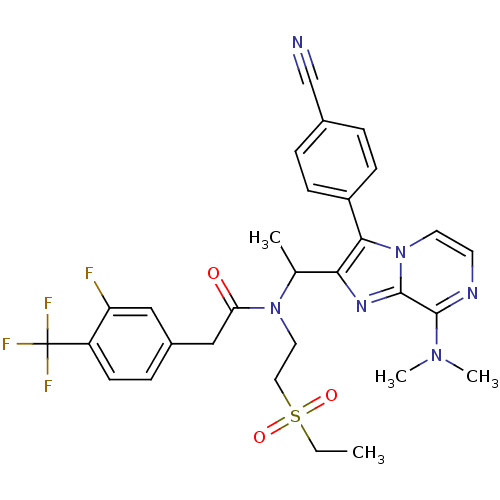 (CHEMBL570857 | rac-N-(1-(3-(4-cyanophenyl)-8-(dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50458169 (Avagacestat | BMS 708163 | BMS-708163 | BMS-708163...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using Notch as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300901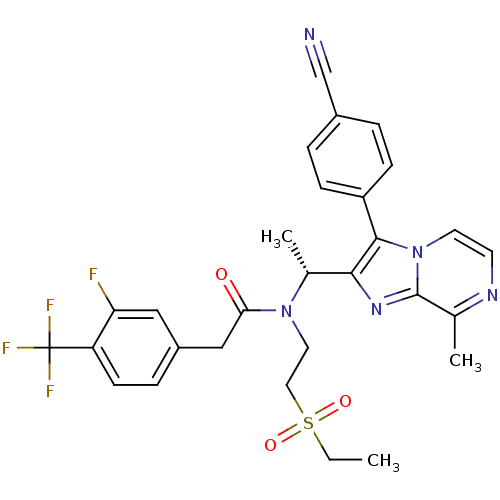 ((R)-N-(1-(3-(4-cyanophenyl)-8-methylimidazo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM28922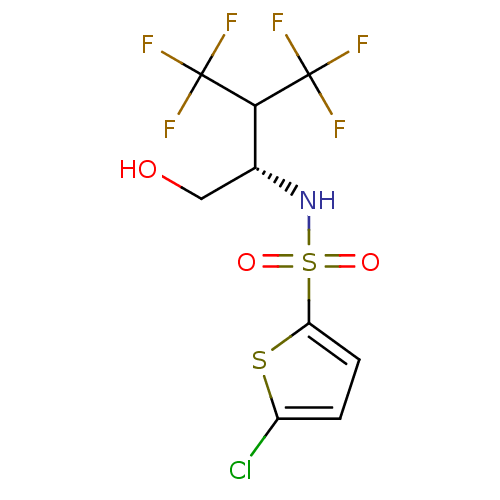 ((2S)-N-(5-chlorothiophen-2-yl)-4,4,4-trifluoro-1-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50300908 (CHEMBL576098 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC | Bioorg Med Chem Lett 19: 5200-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.021 BindingDB Entry DOI: 10.7270/Q2W95980 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50371490 (CHEMBL269932) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]IP10 from human recombinant CXCR3 receptor expressed in IL2-activated human PBMC | Bioorg Med Chem Lett 18: 608-13 (2008) Article DOI: 10.1016/j.bmcl.2007.11.072 BindingDB Entry DOI: 10.7270/Q2668F1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492092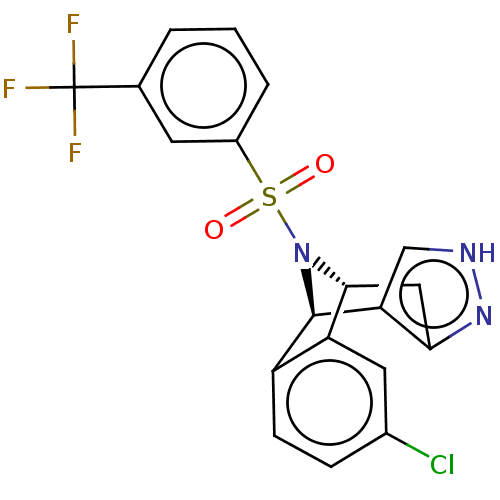 (CHEMBL2396961) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50492084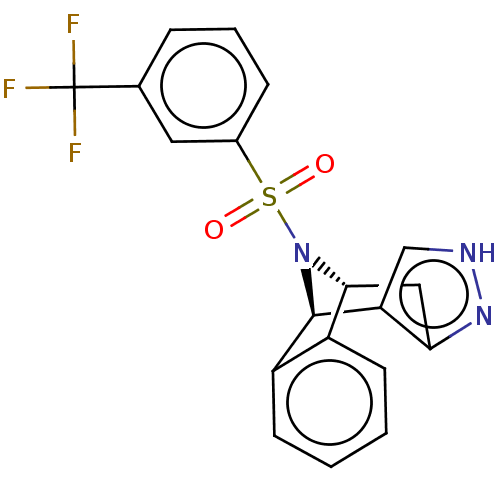 (CHEMBL2396958) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA | Bioorg Med Chem Lett 23: 996-1000 (2013) Article DOI: 10.1016/j.bmcl.2012.12.039 BindingDB Entry DOI: 10.7270/Q2Q81H1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50229383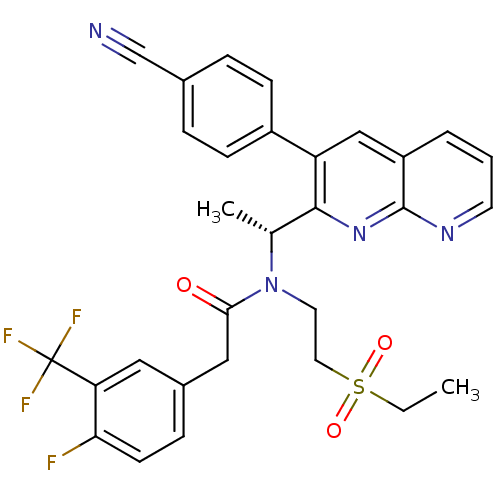 ((R)-N-(1-(3-(4-cyanophenyl)-1,8-naphthyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA | Bioorg Med Chem Lett 18: 688-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.060 BindingDB Entry DOI: 10.7270/Q2WQ03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50352620 (CHEMBL1822151) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 5521-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.100 BindingDB Entry DOI: 10.7270/Q29Z959K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 902 total ) | Next | Last >> |