| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin D |
|---|
| Ligand | BDBM16751 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_462006 (CHEMBL944849) |
|---|
| IC50 | 60000±n/a nM |
|---|
| Citation |  Cole, DC; Stock, JR; Chopra, R; Cowling, R; Ellingboe, JW; Fan, KY; Harrison, BL; Hu, Y; Jacobsen, S; Jennings, LD; Jin, G; Lohse, PA; Malamas, MS; Manas, ES; Moore, WJ; O'Donnell, MM; Olland, AM; Robichaud, AJ; Svenson, K; Wu, J; Wagner, E; Bard, J Acylguanidine inhibitors of beta-secretase: optimization of the pyrrole ring substituents extending into the S1 and S3 substrate binding pockets. Bioorg Med Chem Lett18:1063-6 (2008) [PubMed] Article Cole, DC; Stock, JR; Chopra, R; Cowling, R; Ellingboe, JW; Fan, KY; Harrison, BL; Hu, Y; Jacobsen, S; Jennings, LD; Jin, G; Lohse, PA; Malamas, MS; Manas, ES; Moore, WJ; O'Donnell, MM; Olland, AM; Robichaud, AJ; Svenson, K; Wu, J; Wagner, E; Bard, J Acylguanidine inhibitors of beta-secretase: optimization of the pyrrole ring substituents extending into the S1 and S3 substrate binding pockets. Bioorg Med Chem Lett18:1063-6 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cathepsin D |
|---|
| Name: | Cathepsin D |
|---|
| Synonyms: | CATD_HUMAN | CPSD | CTSD | Cathepsin D [Precursor] | Cathepsin D heavy chain | Cathepsin D light chain | Cathepsin D precursor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 44551.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human proCathepsin D (SwissProt accession number P07339) was expressed in Sf9 cells, purified, and autoactivated. |
|---|
| Residue: | 412 |
|---|
| Sequence: | MQPSSLLPLALCLLAAPASALVRIPLHKFTSIRRTMSEVGGSVEDLIAKGPVSKYSQAVP
AVTEGPIPEVLKNYMDAQYYGEIGIGTPPQCFTVVFDTGSSNLWVPSIHCKLLDIACWIH
HKYNSDKSSTYVKNGTSFDIHYGSGSLSGYLSQDTVSVPCQSASSASALGGVKVERQVFG
EATKQPGITFIAAKFDGILGMAYPRISVNNVLPVFDNLMQQKLVDQNIFSFYLSRDPDAQ
PGGELMLGGTDSKYYKGSLSYLNVTRKAYWQVHLDQVEVASGLTLCKEGCEAIVDTGTSL
MVGPVDEVRELQKAIGAVPLIQGEYMIPCEKVSTLPAITLKLGGKGYKLSPEDYTLKVSQ
AGKTLCLSGFMGMDIPPPSGPLWILGDVFIGRYYTVFDRDNNRVGFAEAARL
|
|
|
|---|
| BDBM16751 |
|---|
| n/a |
|---|
| Name | BDBM16751 |
|---|
| Synonyms: | (N-(diaminomethylene)-2,4-diphenyl-1H-pyrrole-1-acetamide) | Acylguanidine, 7a | CHEMBL217068 | N-(diaminomethylidene)-2-(2,5-diphenyl-1H-pyrrol-1-yl)acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H18N4O |
|---|
| Mol. Mass. | 318.3724 |
|---|
| SMILES | NC(=N)NC(=O)Cn1c(ccc1-c1ccccc1)-c1ccccc1 |
|---|
| Structure | 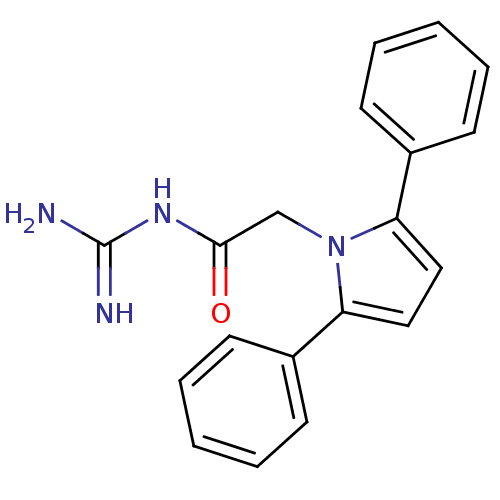
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cole, DC; Stock, JR; Chopra, R; Cowling, R; Ellingboe, JW; Fan, KY; Harrison, BL; Hu, Y; Jacobsen, S; Jennings, LD; Jin, G; Lohse, PA; Malamas, MS; Manas, ES; Moore, WJ; O'Donnell, MM; Olland, AM; Robichaud, AJ; Svenson, K; Wu, J; Wagner, E; Bard, J Acylguanidine inhibitors of beta-secretase: optimization of the pyrrole ring substituents extending into the S1 and S3 substrate binding pockets. Bioorg Med Chem Lett18:1063-6 (2008) [PubMed] Article
Cole, DC; Stock, JR; Chopra, R; Cowling, R; Ellingboe, JW; Fan, KY; Harrison, BL; Hu, Y; Jacobsen, S; Jennings, LD; Jin, G; Lohse, PA; Malamas, MS; Manas, ES; Moore, WJ; O'Donnell, MM; Olland, AM; Robichaud, AJ; Svenson, K; Wu, J; Wagner, E; Bard, J Acylguanidine inhibitors of beta-secretase: optimization of the pyrrole ring substituents extending into the S1 and S3 substrate binding pockets. Bioorg Med Chem Lett18:1063-6 (2008) [PubMed] Article