| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein phosphatase F |
|---|
| Ligand | BDBM50149232 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_490538 (CHEMBL981144) |
|---|
| Ki | 1300±n/a nM |
|---|
| Citation |  Ling, Q; Huang, Y; Zhou, Y; Cai, Z; Xiong, B; Zhang, Y; Ma, L; Wang, X; Li, X; Li, J; Shen, J Illudalic acid as a potential LAR inhibitor: synthesis, SAR, and preliminary studies on the mechanism of action. Bioorg Med Chem16:7399-409 (2008) [PubMed] Article Ling, Q; Huang, Y; Zhou, Y; Cai, Z; Xiong, B; Zhang, Y; Ma, L; Wang, X; Li, X; Li, J; Shen, J Illudalic acid as a potential LAR inhibitor: synthesis, SAR, and preliminary studies on the mechanism of action. Bioorg Med Chem16:7399-409 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein phosphatase F |
|---|
| Name: | Receptor-type tyrosine-protein phosphatase F |
|---|
| Synonyms: | LAR | Leukocyte common antigen related | Leukocyte common antigen related (LAR) | PTPRF | PTPRF_HUMAN | Receptor-type tyrosine-protein phosphatase F | Receptor-type tyrosine-protein phosphatase F (LAR) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 212869.85 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10586 |
|---|
| Residue: | 1907 |
|---|
| Sequence: | MAPEPAPGRTMVPLVPALVMLGLVAGAHGDSKPVFIKVPEDQTGLSGGVASFVCQATGEP
KPRITWMKKGKKVSSQRFEVIEFDDGAGSVLRIQPLRVQRDEAIYECTATNSLGEINTSA
KLSVLEEEQLPPGFPSIDMGPQLKVVEKARTATMLCAAGGNPDPEISWFKDFLPVDPATS
NGRIKQLRSGALQIESSEESDQGKYECVATNSAGTRYSAPANLYVRVRRVAPRFSIPPSS
QEVMPGGSVNLTCVAVGAPMPYVKWMMGAEELTKEDEMPVGRNVLELSNVVRSANYTCVA
ISSLGMIEATAQVTVKALPKPPIDLVVTETTATSVTLTWDSGNSEPVTYYGIQYRAAGTE
GPFQEVDGVATTRYSIGGLSPFSEYAFRVLAVNSIGRGPPSEAVRARTGEQAPSSPPRRV
QARMLSASTMLVQWEPPEEPNGLVRGYRVYYTPDSRRPPNAWHKHNTDAGLLTTVGSLLP
GITYSLRVLAFTAVGDGPPSPTIQVKTQQGVPAQPADFQAEVESDTRIQLSWLLPPQERI
IMYELVYWAAEDEDQQHKVTFDPTSSYTLEDLKPDTLYRFQLAARSDMGVGVFTPTIEAR
TAQSTPSAPPQKVMCVSMGSTTVRVSWVPPPADSRNGVITQYSVAYEAVDGEDRGRHVVD
GISREHSSWDLVGLEKWTEYRVWVRAHTDVGPGPESSPVLVRTDEDVPSGPPRKVEVEPL
NSTAVHVYWKLPVPSKQHGQIRGYQVTYVRLENGEPRGLPIIQDVMLAEAQWRPEESEDY
ETTISGLTPETTYSVTVAAYTTKGDGARSKPKIVTTTGAVPGRPTMMISTTAMNTALLQW
HPPKELPGELLGYRLQYCRADEARPNTIDFGKDDQHFTVTGLHKGTTYIFRLAAKNRAGL
GEEFEKEIRTPEDLPSGFPQNLHVTGLTTSTTELAWDPPVLAERNGRIISYTVVFRDINS
QQELQNITTDTRFTLTGLKPDTTYDIKVRAWTSKGSGPLSPSIQSRTMPVEQVFAKNFRV
AAAMKTSVLLSWEVPDSYKSAVPFKILYNGQSVEVDGHSMRKLIADLQPNTEYSFVLMNR
GSSAGGLQHLVSIRTAPDLLPHKPLPASAYIEDGRFDLSMPHVQDPSLVRWFYIVVVPID
RVGGSMLTPRWSTPEELELDELLEAIEQGGEEQRRRRRQAERLKPYVAAQLDVLPETFTL
GDKKNYRGFYNRPLSPDLSYQCFVLASLKEPMDQKRYASSPYSDEIVVQVTPAQQQEEPE
MLWVTGPVLAVILIILIVIAILLFKRKRTHSPSSKDEQSIGLKDSLLAHSSDPVEMRRLN
YQTPGMRDHPPIPITDLADNIERLKANDGLKFSQEYESIDPGQQFTWENSNLEVNKPKNR
YANVIAYDHSRVILTSIDGVPGSDYINANYIDGYRKQNAYIATQGPLPETMGDFWRMVWE
QRTATVVMMTRLEEKSRVKCDQYWPARGTETCGLIQVTLLDTVELATYTVRTFALHKSGS
SEKRELRQFQFMAWPDHGVPEYPTPILAFLRRVKACNPLDAGPMVVHCSAGVGRTGCFIV
IDAMLERMKHEKTVDIYGHVTCMRSQRNYMVQTEDQYVFIHEALLEAATCGHTEVPARNL
YAHIQKLGQVPPGESVTAMELEFKLLASSKAHTSRFISANLPCNKFKNRLVNIMPYELTR
VCLQPIRGVEGSDYINASFLDGYRQQKAYIATQGPLAESTEDFWRMLWEHNSTIIVMLTK
LREMGREKCHQYWPAERSARYQYFVVDPMAEYNMPQYILREFKVTDARDGQSRTIRQFQF
TDWPEQGVPKTGEGFIDFIGQVHKTKEQFGQDGPITVHCSAGVGRTGVFITLSIVLERMR
YEGVVDMFQTVKTLRTQRPAMVQTEDQYQLCYRAALEYLGSFDHYAT
|
|
|
|---|
| BDBM50149232 |
|---|
| n/a |
|---|
| Name | BDBM50149232 |
|---|
| Synonyms: | 3-(5-(2-acetamido-3-(4-(carboxy-N-(2-carboxyphenyl)formamido))naphthalen-1-yl)propanamido)pentyloxy)-2-naphthoic acid | 3-{5-[2-[4-carboxy(2-carboxyphenyl)carboxamido-1-naphthyl]-1-methylcarboxamido-(1S)-ethylcarboxamido]pentyloxy}-2-naphthoic acid | CHEMBL324968 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C40H37N3O10 |
|---|
| Mol. Mass. | 719.7359 |
|---|
| SMILES | CC(=O)NC(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |
|---|
| Structure | 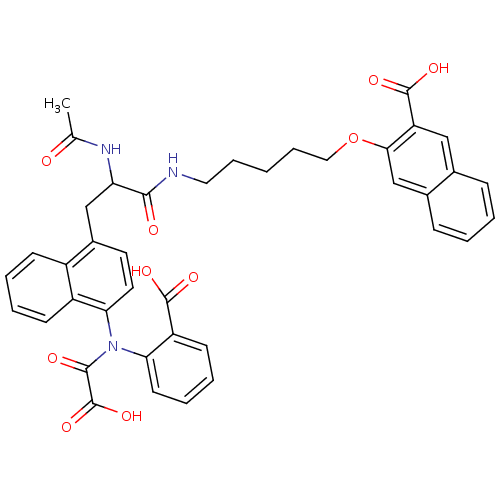
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ling, Q; Huang, Y; Zhou, Y; Cai, Z; Xiong, B; Zhang, Y; Ma, L; Wang, X; Li, X; Li, J; Shen, J Illudalic acid as a potential LAR inhibitor: synthesis, SAR, and preliminary studies on the mechanism of action. Bioorg Med Chem16:7399-409 (2008) [PubMed] Article
Ling, Q; Huang, Y; Zhou, Y; Cai, Z; Xiong, B; Zhang, Y; Ma, L; Wang, X; Li, X; Li, J; Shen, J Illudalic acid as a potential LAR inhibitor: synthesis, SAR, and preliminary studies on the mechanism of action. Bioorg Med Chem16:7399-409 (2008) [PubMed] Article