

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069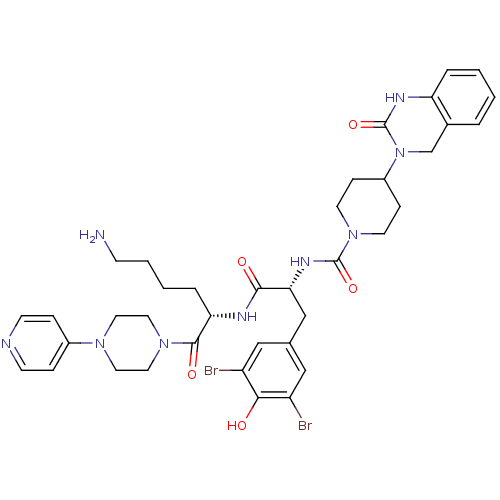 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells | ACS Med Chem Lett 3: 337-341 (2012) Article DOI: 10.1021/ml300021s BindingDB Entry DOI: 10.7270/Q26D5V2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50475411 (CHEMBL197830) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50475407 (CHEMBL198228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50475410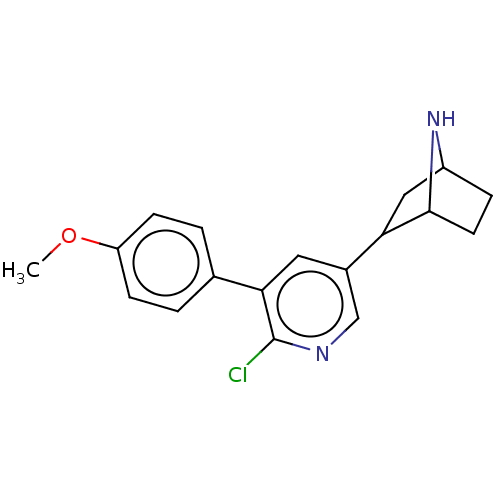 (CHEMBL372133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475411 (CHEMBL197830) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50106490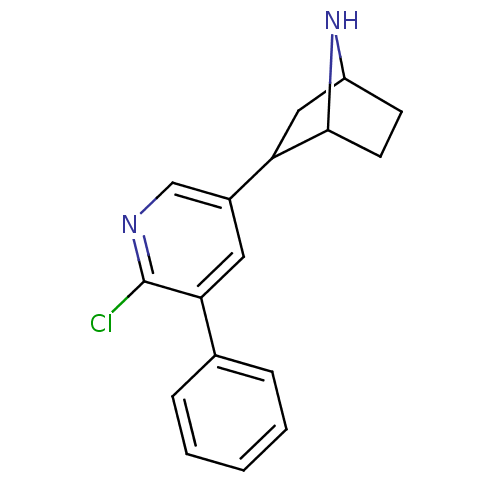 (2-(6-Chloro-5-phenyl-pyridin-3-yl)-7-aza-bicyclo[2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50171007 (4-[5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-2-fluoro-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50106490 (2-(6-Chloro-5-phenyl-pyridin-3-yl)-7-aza-bicyclo[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475410 (CHEMBL372133) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50099821 (4-Bromo-1-methoxy-naphthalene-2-carboxylic acid (9...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D3 expressed in Sf9 cells using [125I]-IABN the radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50099807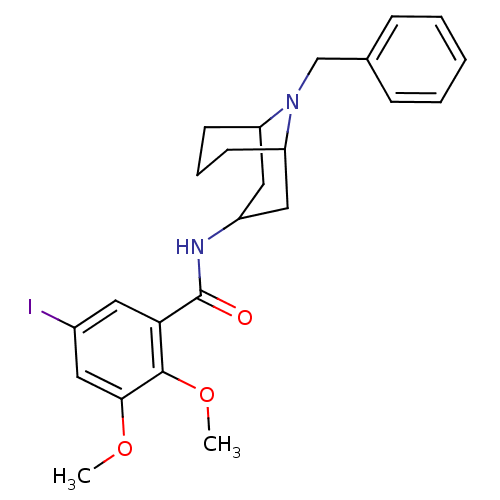 (CHEMBL54866 | N-(9-Benzyl-9-aza-bicyclo[3.3.1]non-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D3 expressed in Sf9 cells using [125I]-IABN the radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475409 (CHEMBL197526) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50099807 (CHEMBL54866 | N-(9-Benzyl-9-aza-bicyclo[3.3.1]non-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 long expressed in Sf9 cells using [125I]-IABN radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475407 (CHEMBL198228) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50475409 (CHEMBL197526) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM86816 (CAS_45263769 | NSC_45263769 | rac-2-(5-(4-chloroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM86816 (CAS_45263769 | NSC_45263769 | rac-2-(5-(4-chloroph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50171007 (4-[5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-2-fluoro-pyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50388882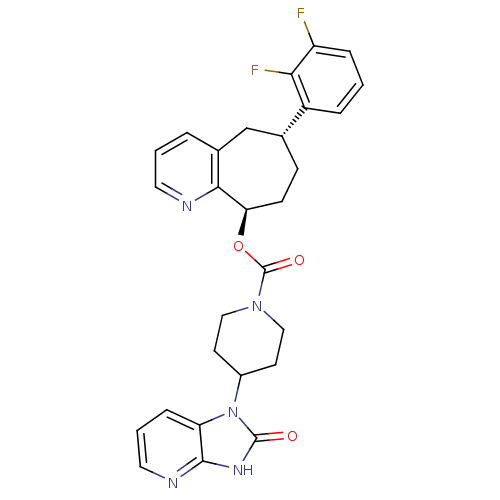 (CHEMBL2063115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells | ACS Med Chem Lett 3: 337-341 (2012) Article DOI: 10.1021/ml300021s BindingDB Entry DOI: 10.7270/Q26D5V2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50382794 (CHEMBL2023191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells | ACS Med Chem Lett 3: 337-341 (2012) Article DOI: 10.1021/ml300021s BindingDB Entry DOI: 10.7270/Q26D5V2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM86815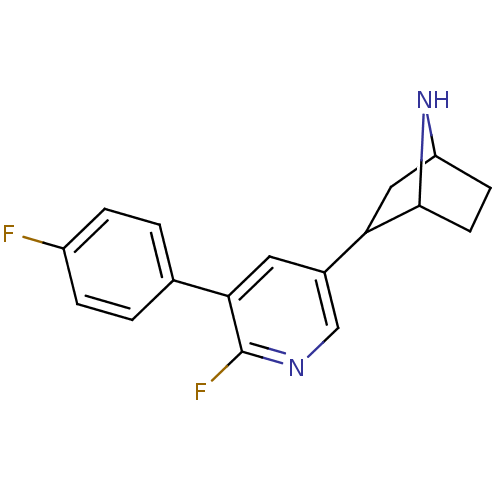 (CAS_45266019 | NSC_45266019 | rac-2-(6-fluoro-5-(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50475411 (CHEMBL197830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM86815 (CAS_45266019 | NSC_45266019 | rac-2-(6-fluoro-5-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta4 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50475410 (CHEMBL372133) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 1 (Homo sapiens (Human)) | BDBM50273370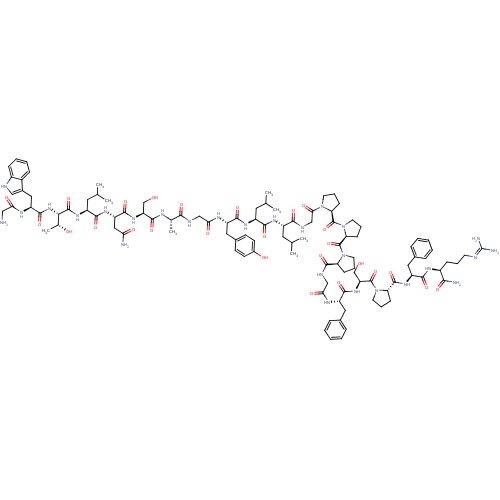 (CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Peptides 19: 1771-81 (1998) Article DOI: 10.1016/s0196-9781(98)00133-8 BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50035105 (1'-{4-[1-(4-fluorophenyl)-1H-3-indolyl]butyl}spiro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangdong Medical University Curated by ChEMBL | Assay Description Binding affinity to sigma-2 receptor (unknown origin) | Eur J Med Chem 147: 227-237 (2018) Article DOI: 10.1016/j.ejmech.2017.11.016 BindingDB Entry DOI: 10.7270/Q2SQ92XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50106490 (2-(6-Chloro-5-phenyl-pyridin-3-yl)-7-aza-bicyclo[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description Affinity towards cloned human 5-hydroxytryptamine 1B receptor | Bioorg Med Chem Lett 15: 4786-9 (2005) Article DOI: 10.1016/j.bmcl.2005.07.024 BindingDB Entry DOI: 10.7270/Q2NP23ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50475407 (CHEMBL198228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236864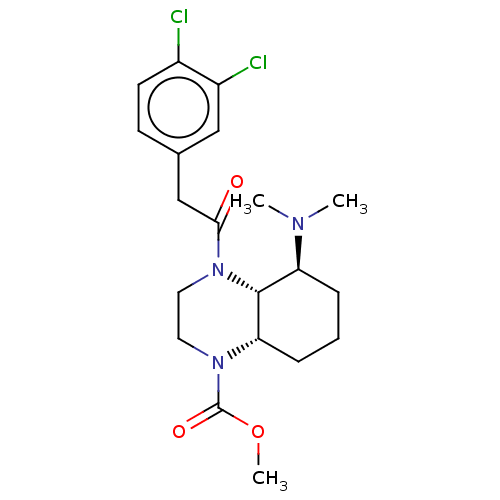 (CHEMBL4096473) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Inhibition of high affinity uptake of [3H]DA using rat nerve endings obtained from brain regions enriched in DAT. | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta4 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50475409 (CHEMBL197526) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50241107 (1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50099794 (4-Bromo-1-methoxy-naphthalene-2-carboxylic acid (1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D3 expressed in Sf9 cells using [125I]-IABN the radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50099821 (4-Bromo-1-methoxy-naphthalene-2-carboxylic acid (9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 long expressed in Sf9 cells using [125I]-IABN radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50020217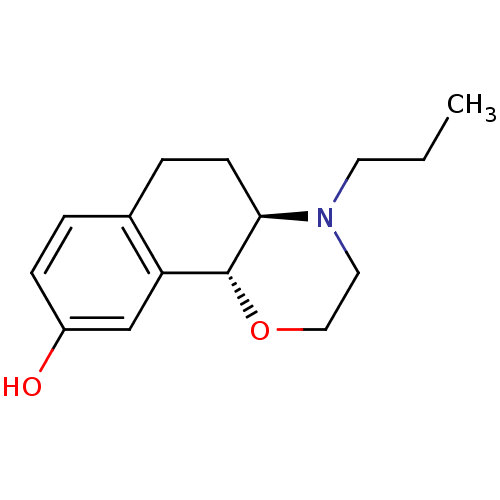 ((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Agonistic activity at DRD3 receptor | Bioorg Med Chem Lett 19: 5056-9 (2009) Article DOI: 10.1016/j.bmcl.2009.07.055 BindingDB Entry DOI: 10.7270/Q2PR7X7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 1 (Homo sapiens (Human)) | BDBM85070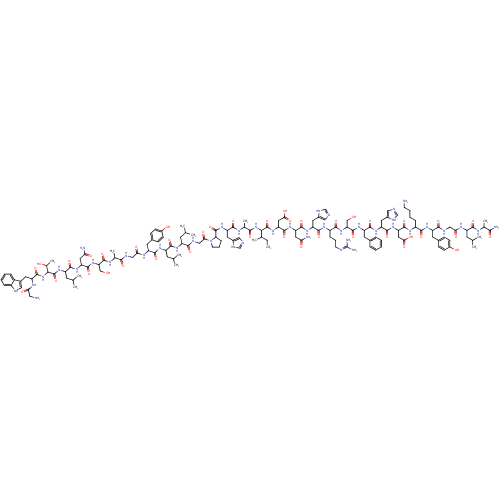 (Galanin, Porcine) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Peptides 19: 1771-81 (1998) Article DOI: 10.1016/s0196-9781(98)00133-8 BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-4 (Rattus norvegicus (Rat)) | BDBM50475407 (CHEMBL198228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta4 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475406 (CHEMBL372326) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130757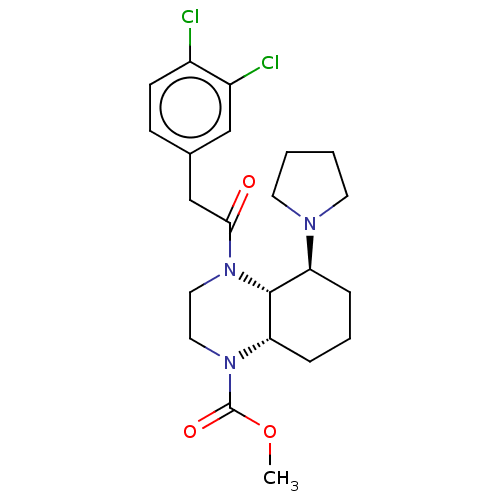 (CHEMBL3634516) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM78433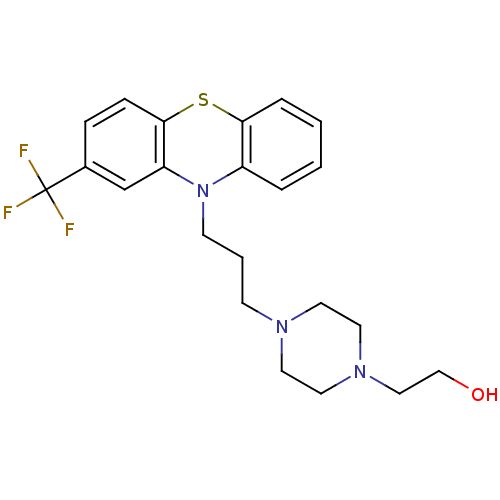 (2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM79172 (2-[4-[(3Z)-3-[2-(trifluoromethyl)-9-thioxanthenyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 1 (Homo sapiens (Human)) | BDBM85073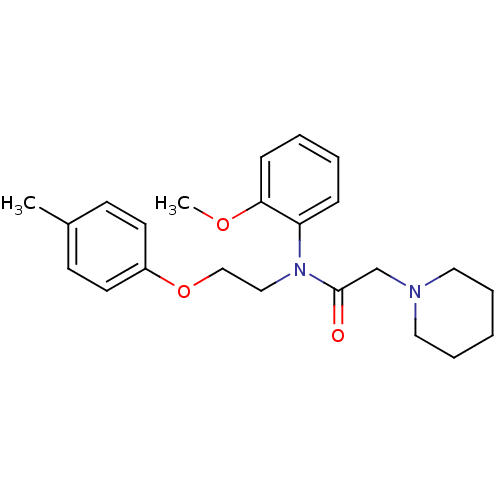 (CAS_3043476 | Galantide (M15) | NSC_3043476) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Peptides 19: 1771-81 (1998) Article DOI: 10.1016/s0196-9781(98)00133-8 BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 1 (Homo sapiens (Human)) | BDBM85168 (M32) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Peptides 19: 1771-81 (1998) Article DOI: 10.1016/s0196-9781(98)00133-8 BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 19759 total ) | Next | Last >> |