| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 11B1, mitochondrial |
|---|
| Ligand | BDBM50272365 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_510155 (CHEMBL1002228) |
|---|
| IC50 | 99±n/a nM |
|---|
| Citation |  Heim, R; Lucas, S; Grombein, CM; Ries, C; Schewe, KE; Negri, M; Müller-Vieira, U; Birk, B; Hartmann, RW Overcoming undesirable CYP1A2 inhibition of pyridylnaphthalene-type aldosterone synthase inhibitors: influence of heteroaryl derivatization on potency and selectivity. J Med Chem51:5064-74 (2008) [PubMed] Article Heim, R; Lucas, S; Grombein, CM; Ries, C; Schewe, KE; Negri, M; Müller-Vieira, U; Birk, B; Hartmann, RW Overcoming undesirable CYP1A2 inhibition of pyridylnaphthalene-type aldosterone synthase inhibitors: influence of heteroaryl derivatization on potency and selectivity. J Med Chem51:5064-74 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 11B1, mitochondrial |
|---|
| Name: | Cytochrome P450 11B1, mitochondrial |
|---|
| Synonyms: | C11B1_HUMAN | CYP11B1 | CYPXIB1 | Cytochrome P450 11B, mitochondrial precursor | Cytochrome P450 11B1 | Cytochrome P450 11B1 (CYP11B1) | Cytochrome P450 11B1, mitochondrial | S11BH |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57591.44 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P15538 |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALRAKAEVCMAVPWLSLQRAQALGTRAARVPRTVLPFEAMPRRPGNRWLRLLQIWREQG
YEDLHLEVHQTFQELGPIFRYDLGGAGMVCVMLPEDVEKLQQVDSLHPHRMSLEPWVAYR
QHRGHKCGVFLLNGPEWRFNRLRLNPEVLSPNAVQRFLPMVDAVARDFSQALKKKVLQNA
RGSLTLDVQPSIFHYTIEASNLALFGERLGLVGHSPSSASLNFLHALEVMFKSTVQLMFM
PRSLSRWTSPKVWKEHFEAWDCIFQYGDNCIQKIYQELAFSRPQQYTSIVAELLLNAELS
PDAIKANSMELTAGSVDTTVFPLLMTLFELARNPNVQQALRQESLAAAASISEHPQKATT
ELPLLRAALKETLRLYPVGLFLERVASSDLVLQNYHIPAGTLVRVFLYSLGRNPALFPRP
ERYNPQRWLDIRGSGRNFYHVPFGFGMRQCLGRRLAEAEMLLLLHHVLKHLQVETLTQED
IKMVYSFILRPSMFPLLTFRAIN
|
|
|
|---|
| BDBM50272365 |
|---|
| n/a |
|---|
| Name | BDBM50272365 |
|---|
| Synonyms: | 1-[5-(6-Methoxynaphthalen-2-yl)-pyridin-3-yl]ethanol | CHEMBL500395 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H17NO2 |
|---|
| Mol. Mass. | 279.3331 |
|---|
| SMILES | COc1ccc2cc(ccc2c1)-c1cncc(c1)C(C)O |
|---|
| Structure | 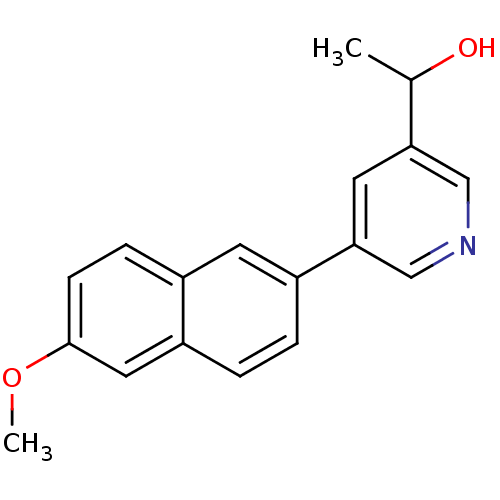
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Heim, R; Lucas, S; Grombein, CM; Ries, C; Schewe, KE; Negri, M; Müller-Vieira, U; Birk, B; Hartmann, RW Overcoming undesirable CYP1A2 inhibition of pyridylnaphthalene-type aldosterone synthase inhibitors: influence of heteroaryl derivatization on potency and selectivity. J Med Chem51:5064-74 (2008) [PubMed] Article
Heim, R; Lucas, S; Grombein, CM; Ries, C; Schewe, KE; Negri, M; Müller-Vieira, U; Birk, B; Hartmann, RW Overcoming undesirable CYP1A2 inhibition of pyridylnaphthalene-type aldosterone synthase inhibitors: influence of heteroaryl derivatization on potency and selectivity. J Med Chem51:5064-74 (2008) [PubMed] Article