

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046071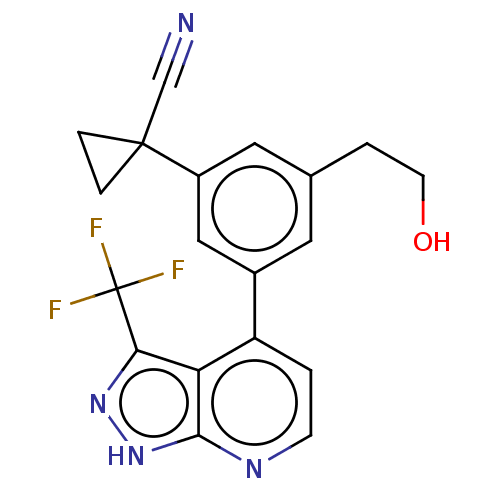 (CHEMBL3310277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046102 (CHEMBL3310293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046097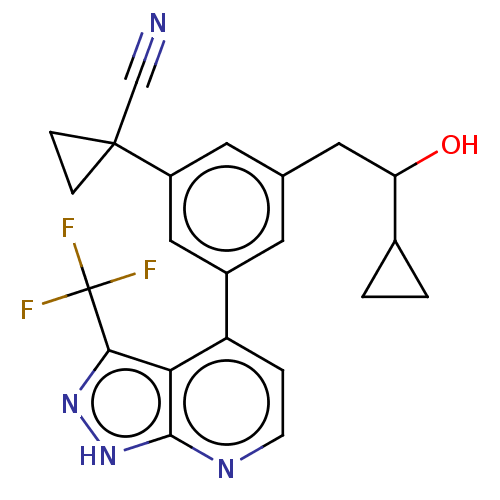 (CHEMBL3310288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046066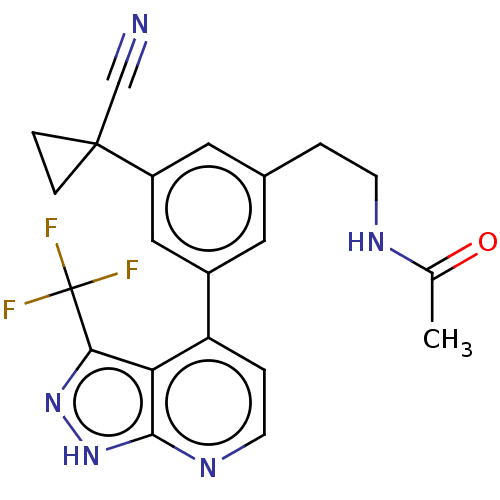 (CHEMBL3310284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046095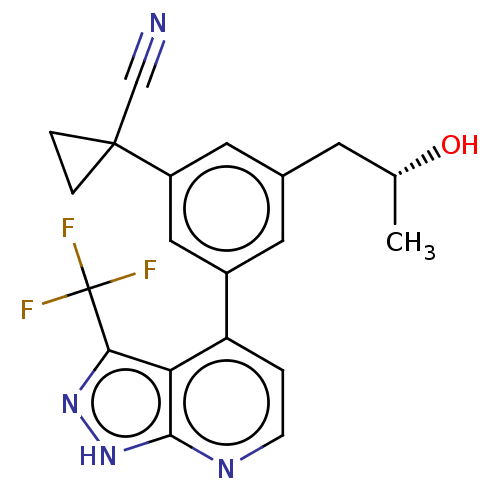 (CHEMBL3310286) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046058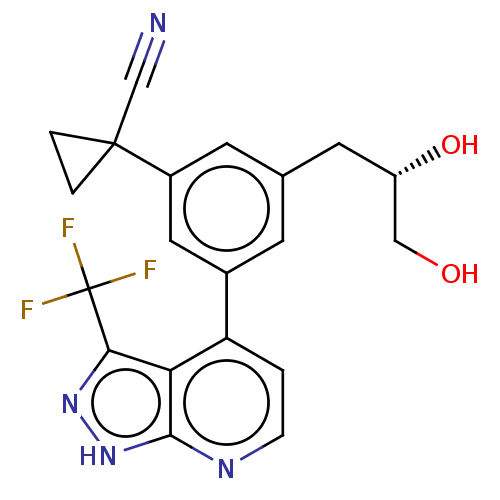 (CHEMBL3310294) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046059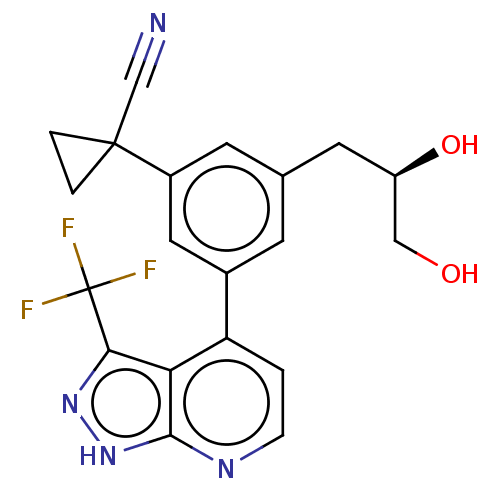 (CHEMBL3310295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046098 (CHEMBL3310289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046069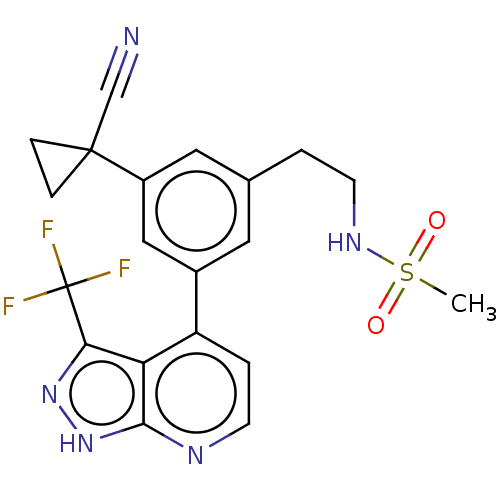 (CHEMBL3310285) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046096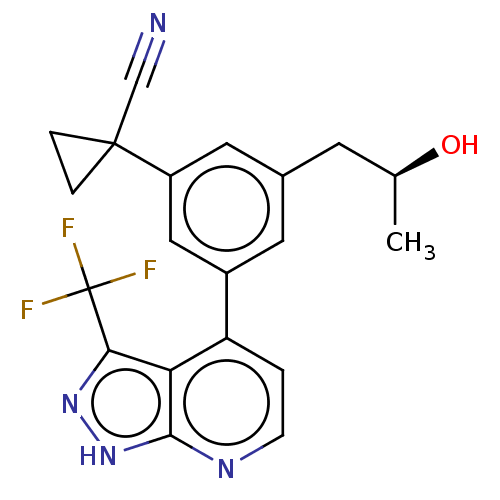 (CHEMBL3310287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046103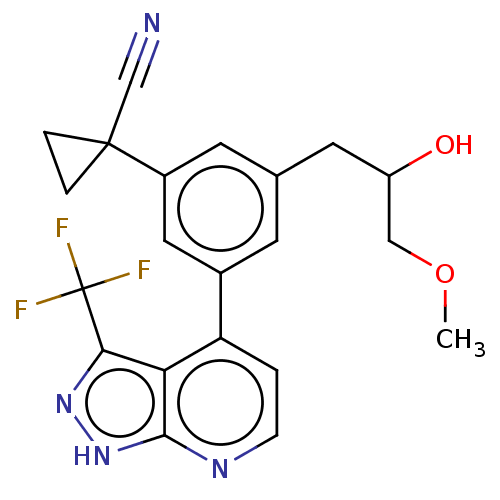 (CHEMBL3310296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046088 (CHEMBL3310278) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046089 (CHEMBL3310279) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046093 (CHEMBL3310282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046092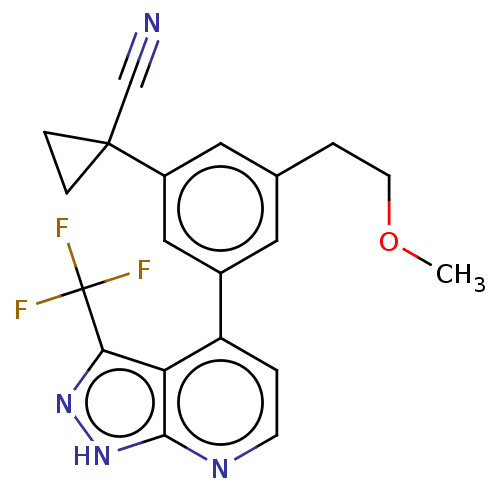 (CHEMBL3310281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046094 (CHEMBL3310283) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046091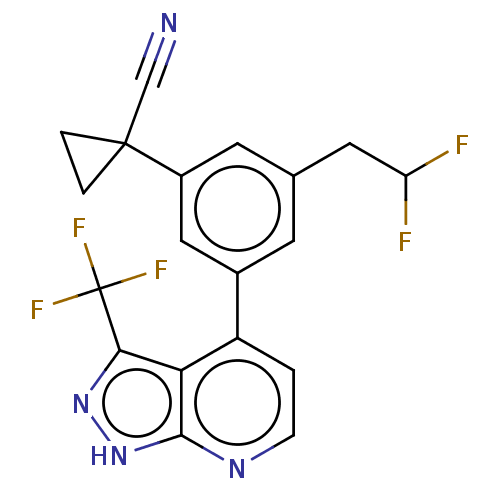 (CHEMBL3310280) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046099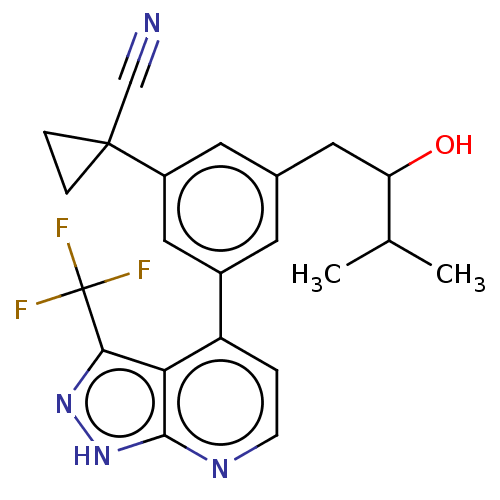 (CHEMBL3310290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046100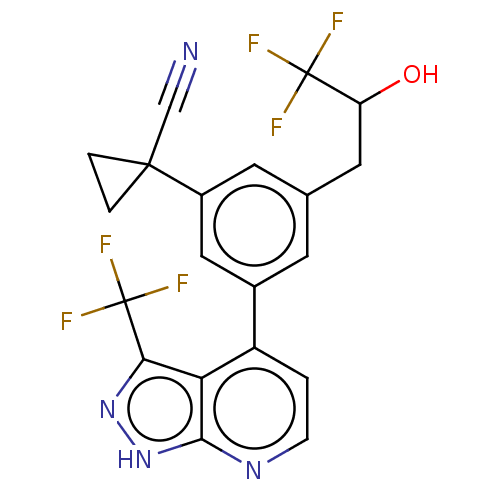 (CHEMBL3310291) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50046101 (CHEMBL3310292) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 446 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKCepsilon (unknown origin) using ERMRPRKRQGSVRRRV peptide substrate by coupled-enzyme assay | Bioorg Med Chem Lett 24: 3398-402 (2014) Article DOI: 10.1016/j.bmcl.2014.05.082 BindingDB Entry DOI: 10.7270/Q27H1M7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227255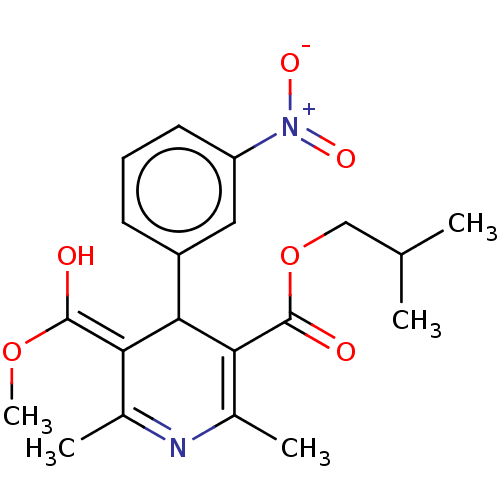 (CHEMBL102907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to guinea pig ileal longitudinal smooth muscle | J Med Chem 31: 300-5 (1988) BindingDB Entry DOI: 10.7270/Q2GM89HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227261 (CHEMBL317044) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to guinea pig ileal longitudinal smooth muscle | J Med Chem 31: 300-5 (1988) BindingDB Entry DOI: 10.7270/Q2GM89HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227259 (CHEMBL441428) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to guinea pig ileal longitudinal smooth muscle | J Med Chem 31: 300-5 (1988) BindingDB Entry DOI: 10.7270/Q2GM89HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227256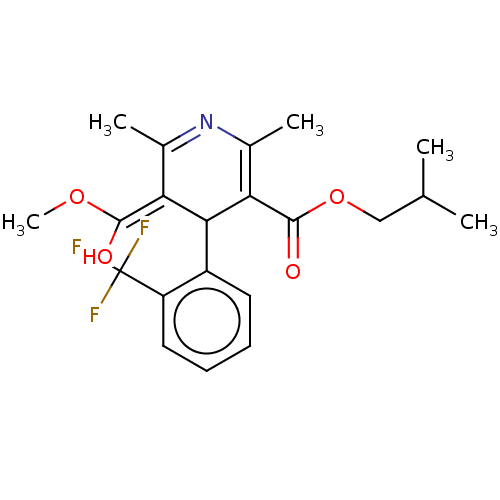 (CHEMBL103806) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to guinea pig ileal longitudinal smooth muscle | J Med Chem 31: 300-5 (1988) BindingDB Entry DOI: 10.7270/Q2GM89HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50273814 (6-Isoquinolin-4-yl-1-methyl-3,4-dihydroquinolin-2(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 8077-87 (2008) Article DOI: 10.1021/jm800888q BindingDB Entry DOI: 10.7270/Q23B6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227257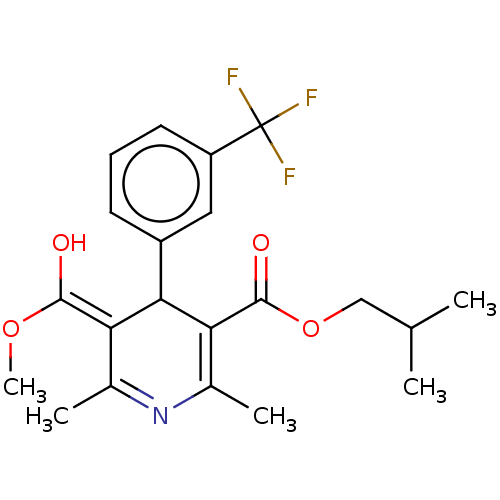 (CHEMBL321999) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to guinea pig ileal longitudinal smooth muscle | J Med Chem 31: 300-5 (1988) BindingDB Entry DOI: 10.7270/Q2GM89HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336520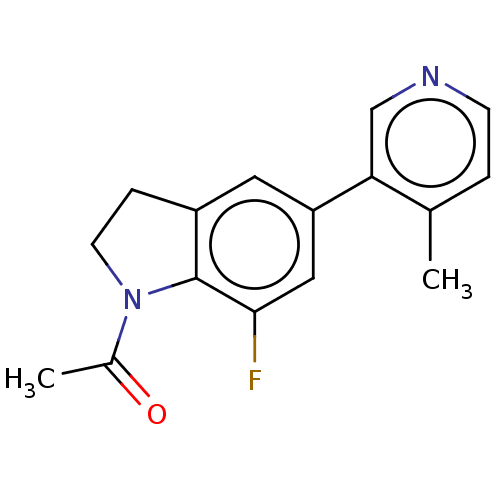 (1-(7-fluoro-5-(4- methylpyridin-3- yl)indolin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp; ElexoPharm GmbH US Patent | Assay Description V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... | US Patent US9745282 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50341470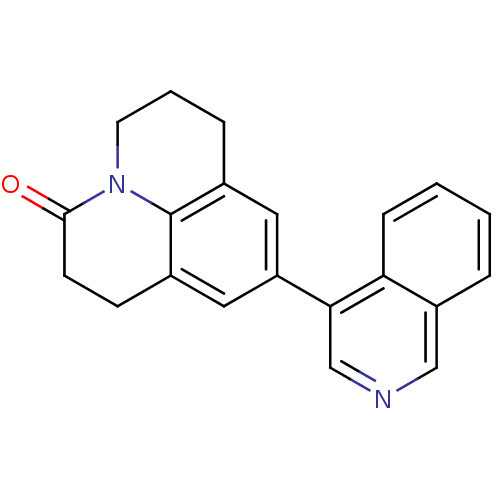 (9-Isoquinolin-4-yl-1,2,6,7-tetrahydro-5H-pyrido[3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells assessed as conversion of [4-14C]-11-deoxycorticosterone substrate by HPTLC assay | J Med Chem 54: 2307-19 (2011) Article DOI: 10.1021/jm101470k BindingDB Entry DOI: 10.7270/Q2765FM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272366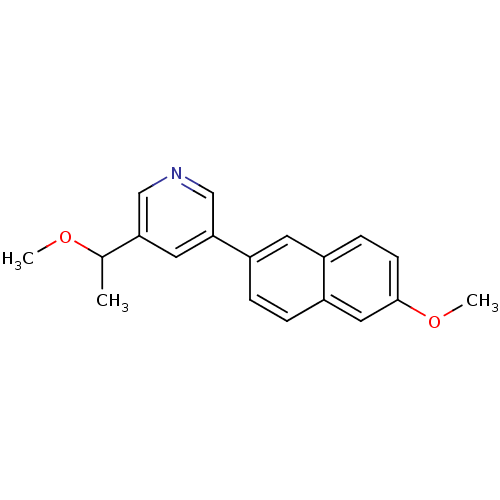 (3-(1-Methoxyethyl)-5-(6-methoxynaphthalen-2-yl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272321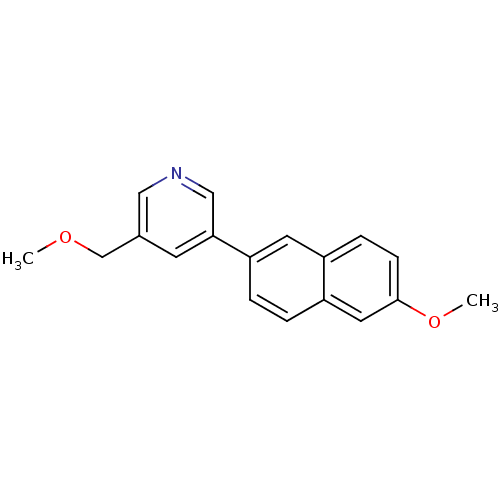 (3-(Methoxymethyl)-5-(6-methoxynaphthalen-2-yl)pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50341469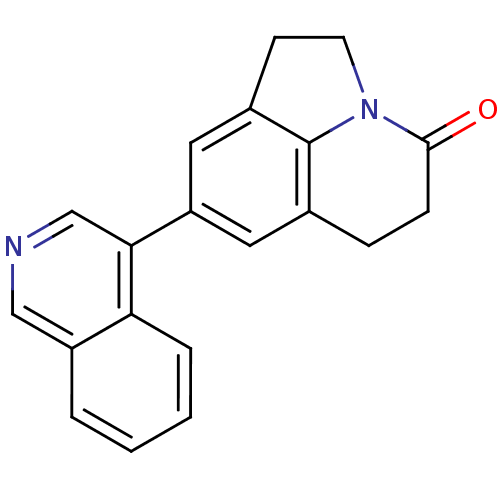 (8-Isoquinolin-4-yl-1,2,5,6-tetrahydro-4H-pyrrolo[3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells assessed as conversion of [4-14C]-11-deoxycorticosterone substrate by HPTLC assay | J Med Chem 54: 2307-19 (2011) Article DOI: 10.1021/jm101470k BindingDB Entry DOI: 10.7270/Q2765FM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50273781 (6-(5-methoxypyridin-3-yl)-1-methyl-3,4-dihydroquin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 8077-87 (2008) Article DOI: 10.1021/jm800888q BindingDB Entry DOI: 10.7270/Q23B6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50273813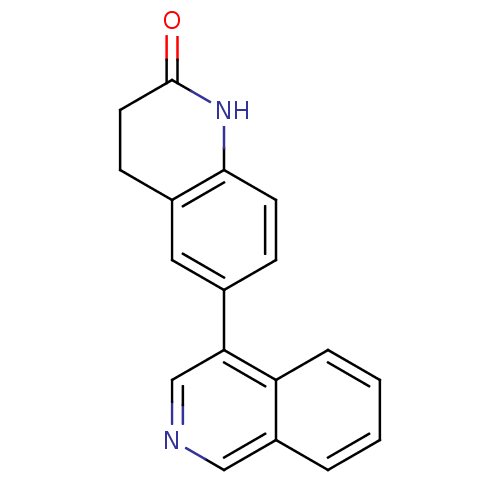 (6-Isoquinolin-4-yl-3,4-dihydroquinolin-2(1H)-one |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 8077-87 (2008) Article DOI: 10.1021/jm800888q BindingDB Entry DOI: 10.7270/Q23B6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227262 (CHEMBL105444) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to guinea pig ileal longitudinal smooth muscle | J Med Chem 31: 300-5 (1988) BindingDB Entry DOI: 10.7270/Q2GM89HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50112984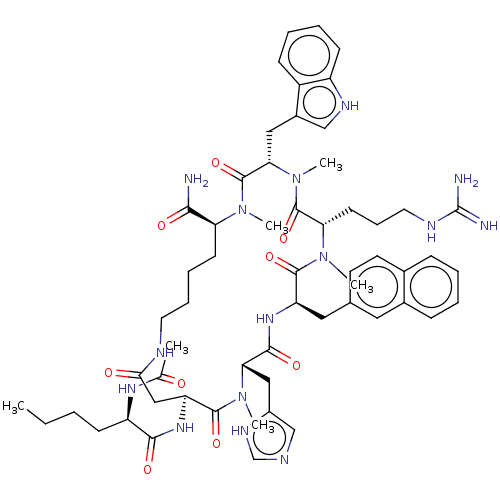 (CHEMBL3601428) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle4,D-Phe7]-alpha-MSH from human MC4 receptor expressed in HEK293 cells after 40 mins by luminescence counting | J Med Chem 58: 6359-67 (2015) Article DOI: 10.1021/acs.jmedchem.5b00102 BindingDB Entry DOI: 10.7270/Q251410F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272365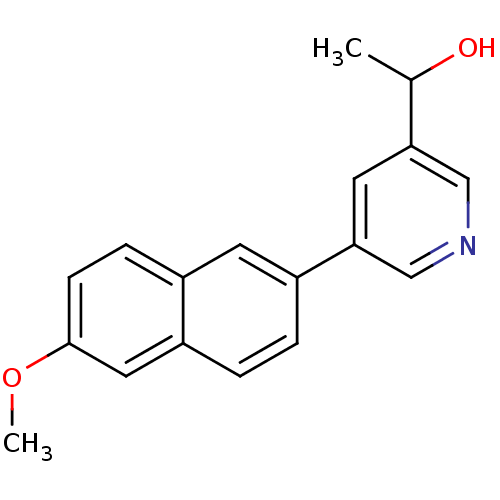 (1-[5-(6-Methoxynaphthalen-2-yl)-pyridin-3-yl]ethan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272370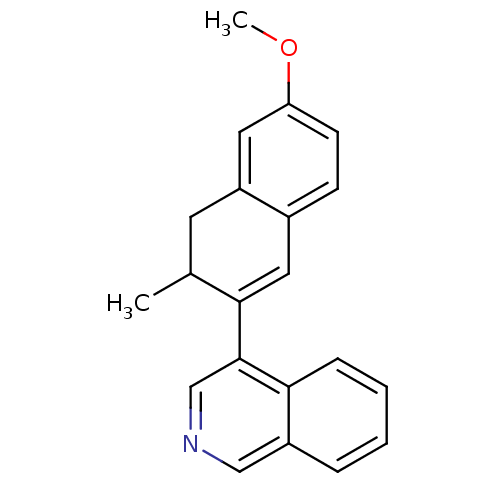 (4-(6-Methoxy-3-methyl-3,4-dihydronaphthalen-2-yl)i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50019871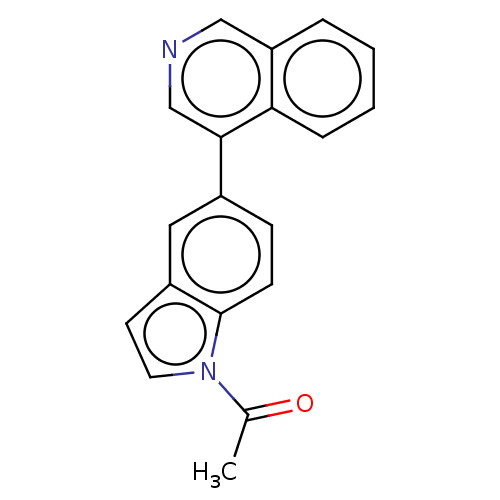 (CHEMBL3287192 | US9745282, 51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp; ElexoPharm GmbH US Patent | Assay Description V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... | US Patent US9745282 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272368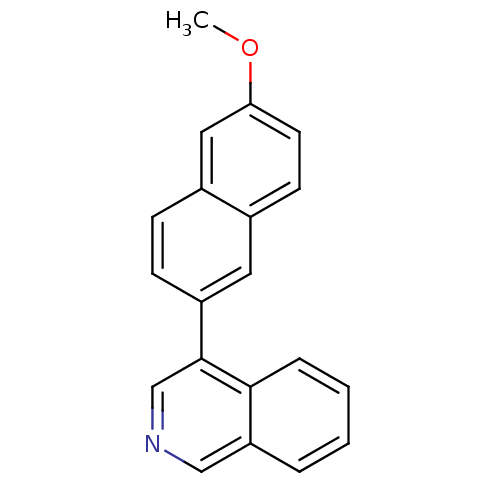 (4-(6-Methoxynaphthalen-2-yl)isoquinoline | CHEMBL5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50341462 (8-(5-Methoxypyridin-3-yl)-1,2,5,6-tetrahydro-4H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells assessed as conversion of [4-14C]-11-deoxycorticosterone substrate by HPTLC assay | J Med Chem 54: 2307-19 (2011) Article DOI: 10.1021/jm101470k BindingDB Entry DOI: 10.7270/Q2765FM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272247 (6-(4-Methylpyridin-3-yl)-2-naphthonitrile | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336320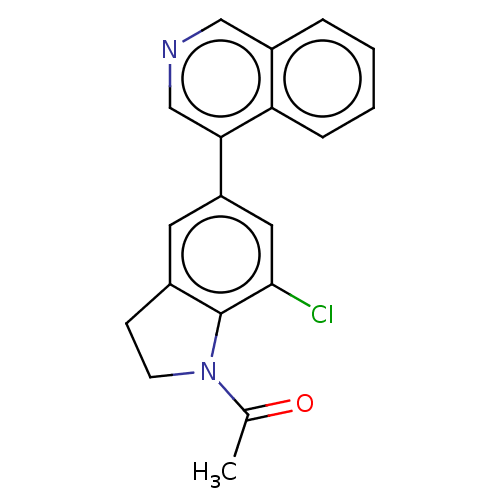 (1-(7-chloro-5- (isoquinolin-4-yl) indolin-1- yl)et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp; ElexoPharm GmbH US Patent | Assay Description V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... | US Patent US9745282 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50341474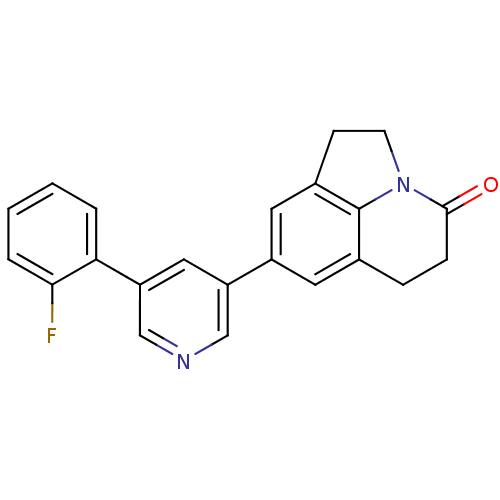 (8-[5-(2-Fluorophenyl)pyridin-3-yl]-1,2,5,6-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells assessed as conversion of [4-14C]-11-deoxycorticosterone substrate by HPTLC assay | J Med Chem 54: 2307-19 (2011) Article DOI: 10.1021/jm101470k BindingDB Entry DOI: 10.7270/Q2765FM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50019886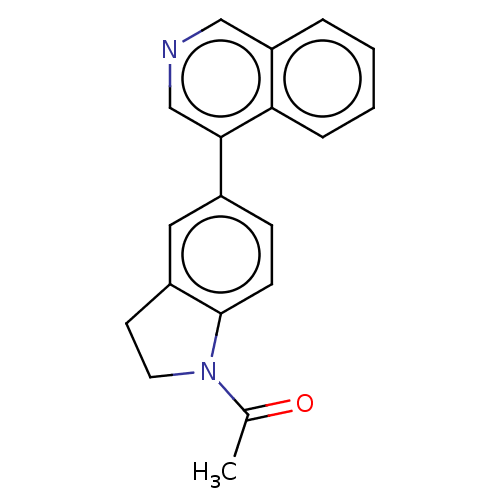 (CHEMBL3287178 | US9745282, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp; ElexoPharm GmbH US Patent | Assay Description V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... | US Patent US9745282 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM336516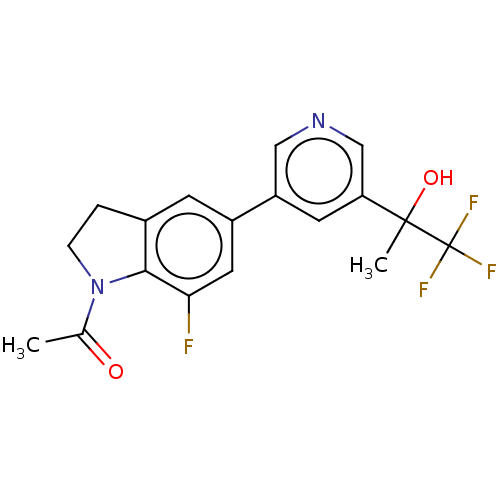 ((R)-1-(7-fluoro-5-(5- (1,1,1-trifluoro-2- hydroxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp; ElexoPharm GmbH US Patent | Assay Description V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... | US Patent US9745282 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272289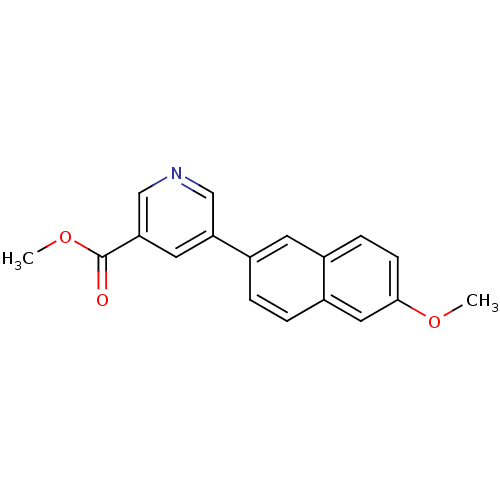 (CHEMBL500146 | Methyl 5-(6-methoxynaphthalen-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50272246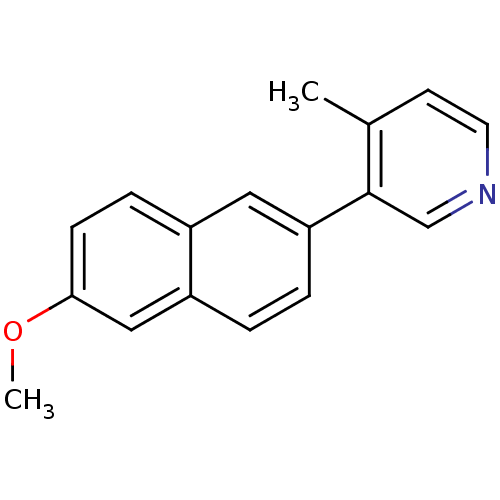 (3-(6-Methoxynaphthalen-2-yl)-4-methylpyridine | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells | J Med Chem 51: 5064-74 (2008) Article DOI: 10.1021/jm800377h BindingDB Entry DOI: 10.7270/Q2Z89C75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZ cells using [3H]-11-deoxycorticosterone as substrate after 1 hr by HPLC analysis | Eur J Med Chem 89: 597-605 (2014) Article DOI: 10.1016/j.ejmech.2014.10.027 BindingDB Entry DOI: 10.7270/Q2X068N3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50341476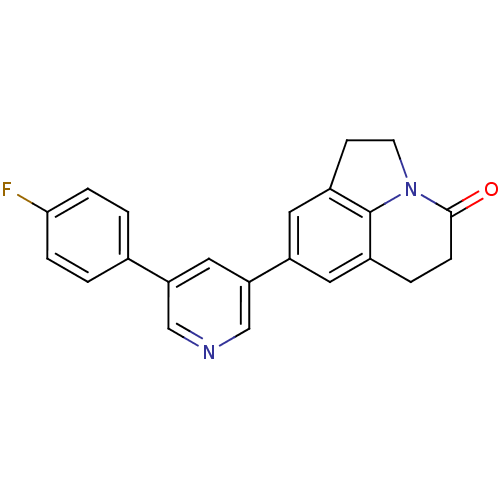 (8-[5-(4-Fluorophenyl)-pyridin-3-yl]-1,2,5,6-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells assessed as conversion of [4-14C]-11-deoxycorticosterone substrate by HPTLC assay | J Med Chem 54: 2307-19 (2011) Article DOI: 10.1021/jm101470k BindingDB Entry DOI: 10.7270/Q2765FM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50341463 (9-(5-Methoxypyridin-3-yl)-1,2,6,7-tetrahydro-5H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79 MZh cells assessed as conversion of [4-14C]-11-deoxycorticosterone substrate by HPTLC assay | J Med Chem 54: 2307-19 (2011) Article DOI: 10.1021/jm101470k BindingDB Entry DOI: 10.7270/Q2765FM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 857 total ) | Next | Last >> |