| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Muscarinic acetylcholine receptor M4 |
|---|
| Ligand | BDBM50275588 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_492760 (CHEMBL939448) |
|---|
| EC50 | 9.1±n/a nM |
|---|
| Citation |  Miller, NR; Daniels, RN; Bridges, TM; Brady, AE; Conn, PJ; Lindsley, CW Synthesis and SAR of analogs of the M1 allosteric agonist TBPB. Part II: Amides, sulfonamides and ureas--the effect of capping the distal basic piperidine nitrogen. Bioorg Med Chem Lett18:5443-7 (2008) [PubMed] Article Miller, NR; Daniels, RN; Bridges, TM; Brady, AE; Conn, PJ; Lindsley, CW Synthesis and SAR of analogs of the M1 allosteric agonist TBPB. Part II: Amides, sulfonamides and ureas--the effect of capping the distal basic piperidine nitrogen. Bioorg Med Chem Lett18:5443-7 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Muscarinic acetylcholine receptor M4 |
|---|
| Name: | Muscarinic acetylcholine receptor M4 |
|---|
| Synonyms: | ACM4_HUMAN | CHRM4 | Cholinergic, muscarinic M4 | Muscarinic acetylcholine receptor | Muscarinic acetylcholine receptor M2 and M4 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 53079.31 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Cholinergic, muscarinic M4 CHRM4 HUMAN M3::P08173 |
|---|
| Residue: | 479 |
|---|
| Sequence: | MANFTPVNGSSGNQSVRLVTSSSHNRYETVEMVFIATVTGSLSLVTVVGNILVMLSIKVN
RQLQTVNNYFLFSLACADLIIGAFSMNLYTVYIIKGYWPLGAVVCDLWLALDYVVSNASV
MNLLIISFDRYFCVTKPLTYPARRTTKMAGLMIAAAWVLSFVLWAPAILFWQFVVGKRTV
PDNQCFIQFLSNPAVTFGTAIAAFYLPVVIMTVLYIHISLASRSRVHKHRPEGPKEKKAK
TLAFLKSPLMKQSVKKPPPGEAAREELRNGKLEEAPPPALPPPPRPVADKDTSNESSSGS
ATQNTKERPATELSTTEATTPAMPAPPLQPRALNPASRWSKIQIVTKQTGNECVTAIEIV
PATPAGMRPAANVARKFASIARNQVRKKRQMAARERKVTRTIFAILLAFILTWTPYNVMV
LVNTFCQSCIPDTVWSIGYWLCYVNSTINPACYALCNATFKKTFRHLLLCQYRNIGTAR
|
|
|
|---|
| BDBM50275588 |
|---|
| n/a |
|---|
| Name | BDBM50275588 |
|---|
| Synonyms: | 2-(4-(2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)-1,4'-bipiperidin-1'-yl)ethyl carbamate | CHEMBL485913 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H29N5O3 |
|---|
| Mol. Mass. | 387.476 |
|---|
| SMILES | NC(=O)OCCN1CCC(CC1)N1CCC(CC1)n1c2ccccc2[nH]c1=O |
|---|
| Structure | 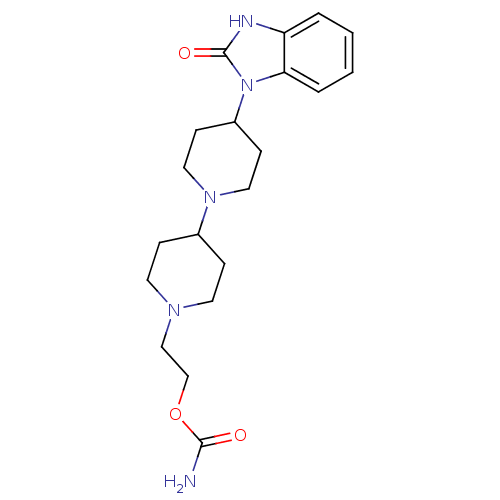
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Miller, NR; Daniels, RN; Bridges, TM; Brady, AE; Conn, PJ; Lindsley, CW Synthesis and SAR of analogs of the M1 allosteric agonist TBPB. Part II: Amides, sulfonamides and ureas--the effect of capping the distal basic piperidine nitrogen. Bioorg Med Chem Lett18:5443-7 (2008) [PubMed] Article
Miller, NR; Daniels, RN; Bridges, TM; Brady, AE; Conn, PJ; Lindsley, CW Synthesis and SAR of analogs of the M1 allosteric agonist TBPB. Part II: Amides, sulfonamides and ureas--the effect of capping the distal basic piperidine nitrogen. Bioorg Med Chem Lett18:5443-7 (2008) [PubMed] Article