| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 beta |
|---|
| Ligand | BDBM50278836 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_565656 (CHEMBL959252) |
|---|
| IC50 | 20000±n/a nM |
|---|
| Citation |  Seefeld, MA; Rouse, MB; McNulty, KC; Sun, L; Wang, J; Yamashita, DS; Luengo, JI; Zhang, S; Minthorn, EA; Concha, NO; Heerding, DA Discovery of 5-pyrrolopyridinyl-2-thiophenecarboxamides as potent AKT kinase inhibitors. Bioorg Med Chem Lett19:2244-8 (2009) [PubMed] Article Seefeld, MA; Rouse, MB; McNulty, KC; Sun, L; Wang, J; Yamashita, DS; Luengo, JI; Zhang, S; Minthorn, EA; Concha, NO; Heerding, DA Discovery of 5-pyrrolopyridinyl-2-thiophenecarboxamides as potent AKT kinase inhibitors. Bioorg Med Chem Lett19:2244-8 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 beta |
|---|
| Name: | Glycogen synthase kinase-3 beta |
|---|
| Synonyms: | GSK-3 beta | GSK-3, beta | GSK3B | GSK3B_HUMAN | Glycogen synthase kinase 3 beta (GSK3B) | Glycogen synthase kinase 3-beta (GSK3B) | Glycogen synthase kinase-3 beta (GSK-3B) | Glycogen synthase kinase-3 beta (GSK3 Beta) | Glycogen synthase kinase-3 beta (GSK3B) | Glycogen synthase kinase-3B (GSK-3B) | Glycogen synthase kinase-3beta (GSK3B) | Serine/threonine-protein kinase GSK3B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46756.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49841 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MSGRPRTTSFAESCKPVQQPSAFGSMKVSRDKDGSKVTTVVATPGQGPDRPQEVSYTDTK
VIGNGSFGVVYQAKLCDSGELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSG
EKKDEVYLNLVLDYVPETVYRVARHYSRAKQTLPVIYVKLYMYQLFRSLAYIHSFGICHR
DIKPQNLLLDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDV
WSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHP
WTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELRDPNVKLPNGRDTPALF
NFTTQELSSNPPLATILIPPHARIQAAASTPTNATAASDANTGDRGQTNNAASASASNST
|
|
|
|---|
| BDBM50278836 |
|---|
| n/a |
|---|
| Name | BDBM50278836 |
|---|
| Synonyms: | CHEMBL523586 | N-((S)-1-amino-3-(3-fluorophenyl)propan-2-yl)-4-bromo-5-(1H-pyrrolo[2,3-b]pyridin-4-yl)thiophene-2-carboxamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H18BrFN4OS |
|---|
| Mol. Mass. | 473.361 |
|---|
| SMILES | NC[C@H](Cc1cccc(F)c1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| |
|---|
| Structure | 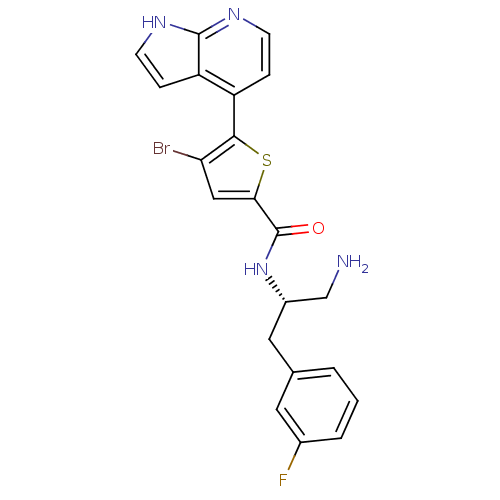
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Seefeld, MA; Rouse, MB; McNulty, KC; Sun, L; Wang, J; Yamashita, DS; Luengo, JI; Zhang, S; Minthorn, EA; Concha, NO; Heerding, DA Discovery of 5-pyrrolopyridinyl-2-thiophenecarboxamides as potent AKT kinase inhibitors. Bioorg Med Chem Lett19:2244-8 (2009) [PubMed] Article
Seefeld, MA; Rouse, MB; McNulty, KC; Sun, L; Wang, J; Yamashita, DS; Luengo, JI; Zhang, S; Minthorn, EA; Concha, NO; Heerding, DA Discovery of 5-pyrrolopyridinyl-2-thiophenecarboxamides as potent AKT kinase inhibitors. Bioorg Med Chem Lett19:2244-8 (2009) [PubMed] Article