| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50280632 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_29087 |
|---|
| IC50 | 5400±n/a nM |
|---|
| Citation |  Shutske, GM; Bores, GM; Bradshaw, KC; Huger, FP; Kapples, KJ; Larsen, RD; Rush, DK; Tomer, JD Synthesis and biological activity of putative mono-hydroxylated metabolites of velnacrine Bioorg Med Chem Lett2:865-870 (1992) Article Shutske, GM; Bores, GM; Bradshaw, KC; Huger, FP; Kapples, KJ; Larsen, RD; Rush, DK; Tomer, JD Synthesis and biological activity of putative mono-hydroxylated metabolites of velnacrine Bioorg Med Chem Lett2:865-870 (1992) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_RAT | Acetylcholinesterase (AChE) | Acetylcholinesterase and butyrylcholinesterase (AChE and BChE) | Acetylcholinesterase precursor | Acetylcholinesterase, AChE | Ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 68193.62 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | P37136 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPWYPLHTPSLASPLLFLLLSLLGGGARAEGREDPQLLVRVRGGQLRGIRLKAPGGPV
SAFLGIPFAEPPVGSRRFMPPEPKRPWSGILDATTFQNVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLIWIYGGGFYSGASSLDVYDGRFLAQVEGTVLVSM
NYRVGTFGFLALPGSREAPGNVGLLDQRLALQWVQENIAAFGGDPMSVTLFGESAGAASV
GMHILSLPSRSLFHRAVLQSGTPNGPWATVSAGEARRRATLLARLVGCPPGGAGGNDTEL
ISCLRTRPAQDLVDHEWHVLPQESIFRFSFVPVVDGDFLSDTPDALINTGDFQDLQVLVG
VVKDEGSYFLVYGVPGFSKDNESLISRAQFLAGVRIGVPQASDLAAEAVVLHYTDWLHPE
DPAHLRDAMSAVVGDHNVVCPVAQLAGRLAAQGARVYAYIFEHRASTLTWPLWMGVPHGY
EIEFIFGLPLDPSLNYTVEERIFAQRLMQYWTNFARTGDPNDPRDSKSPRWPPYTTAAQQ
YVSLNLKPLEVRRGLRAQTCAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQERCSDL
|
|
|
|---|
| BDBM50280632 |
|---|
| n/a |
|---|
| Name | BDBM50280632 |
|---|
| Synonyms: | (1S,3S)-9-Amino-1,2,3,4-tetrahydro-acridine-1,3-diol | CHEMBL354304 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H14N2O2 |
|---|
| Mol. Mass. | 230.2625 |
|---|
| SMILES | Nc1c2[C@@H](O)C[C@@H](O)Cc2nc2ccccc12 |
|---|
| Structure | 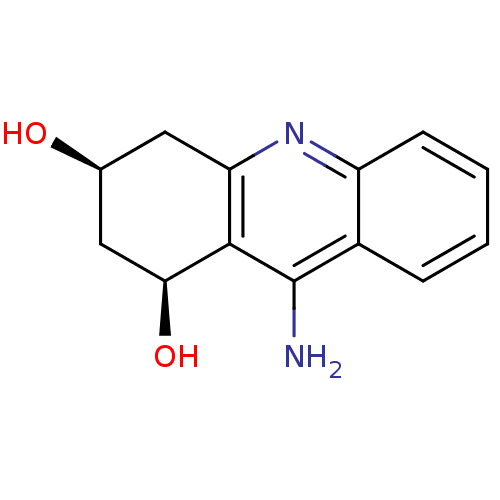
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shutske, GM; Bores, GM; Bradshaw, KC; Huger, FP; Kapples, KJ; Larsen, RD; Rush, DK; Tomer, JD Synthesis and biological activity of putative mono-hydroxylated metabolites of velnacrine Bioorg Med Chem Lett2:865-870 (1992) Article
Shutske, GM; Bores, GM; Bradshaw, KC; Huger, FP; Kapples, KJ; Larsen, RD; Rush, DK; Tomer, JD Synthesis and biological activity of putative mono-hydroxylated metabolites of velnacrine Bioorg Med Chem Lett2:865-870 (1992) Article