| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glutathione reductase, mitochondrial |
|---|
| Ligand | BDBM50300754 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_589949 (CHEMBL1050857) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glutathione reductase, mitochondrial |
|---|
| Name: | Glutathione reductase, mitochondrial |
|---|
| Synonyms: | GLUR | GRD1 | GSHR_HUMAN | GSR | Glutathione reductase | Glutathione reductase (GR) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 56271.52 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00390 |
|---|
| Residue: | 522 |
|---|
| Sequence: | MALLPRALSAGAGPSWRRAARAFRGFLLLLPEPAALTRALSRAMACRQEPQPQGPPPAAG
AVASYDYLVIGGGSGGLASARRAAELGARAAVVESHKLGGTCVNVGCVPKKVMWNTAVHS
EFMHDHADYGFPSCEGKFNWRVIKEKRDAYVSRLNAIYQNNLTKSHIEIIRGHAAFTSDP
KPTIEVSGKKYTAPHILIATGGMPSTPHESQIPGASLGITSDGFFQLEELPGRSVIVGAG
YIAVEMAGILSALGSKTSLMIRHDKVLRSFDSMISTNCTEELENAGVEVLKFSQVKEVKK
TLSGLEVSMVTAVPGRLPVMTMIPDVDCLLWAIGRVPNTKDLSLNKLGIQTDDKGHIIVD
EFQNTNVKGIYAVGDVCGKALLTPVAIAAGRKLAHRLFEYKEDSKLDYNNIPTVVFSHPP
IGTVGLTEDEAIHKYGIENVKTYSTSFTPMYHAVTKRKTKCVMKMVCANKEEKVVGIHMQ
GLGCDEMLQGFAVAVKMGATKADFDNTVAIHPTSSEELVTLR
|
|
|
|---|
| BDBM50300754 |
|---|
| n/a |
|---|
| Name | BDBM50300754 |
|---|
| Synonyms: | 2-Oxy-4-phenyl-furazan-3-carboxylic acid amide | 3-carbamoyl-4-phenyl-1,2,5-oxadiazole 2-oxide | CHEMBL500868 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C9H7N3O3 |
|---|
| Mol. Mass. | 205.1702 |
|---|
| SMILES | NC(=O)c1c(no[n+]1[O-])-c1ccccc1 |
|---|
| Structure | 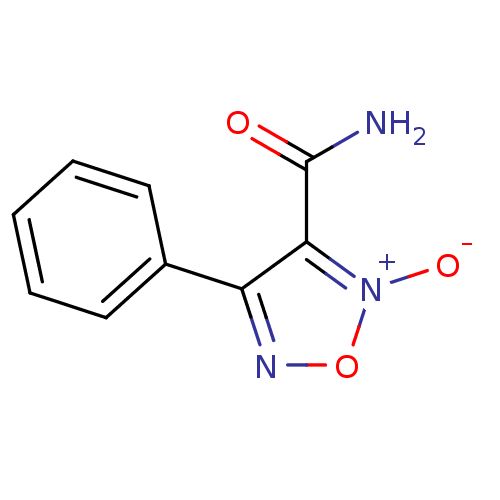
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article
Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article