| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C9 |
|---|
| Ligand | BDBM50307942 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_608358 (CHEMBL1074392) |
|---|
| IC50 | 1000±n/a nM |
|---|
| Citation |  McHardy, T; Caldwell, JJ; Cheung, KM; Hunter, LJ; Taylor, K; Rowlands, M; Ruddle, R; Henley, A; de Haven Brandon, A; Valenti, M; Davies, TG; Fazal, L; Seavers, L; Raynaud, FI; Eccles, SA; Aherne, GW; Garrett, MD; Collins, I Discovery of 4-amino-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamides as selective, orally active inhibitors of protein kinase B (Akt). J Med Chem53:2239-49 (2010) [PubMed] Article McHardy, T; Caldwell, JJ; Cheung, KM; Hunter, LJ; Taylor, K; Rowlands, M; Ruddle, R; Henley, A; de Haven Brandon, A; Valenti, M; Davies, TG; Fazal, L; Seavers, L; Raynaud, FI; Eccles, SA; Aherne, GW; Garrett, MD; Collins, I Discovery of 4-amino-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamides as selective, orally active inhibitors of protein kinase B (Akt). J Med Chem53:2239-49 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C9 |
|---|
| Name: | Cytochrome P450 2C9 |
|---|
| Synonyms: | (R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55636.33 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11712 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKV
YGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKW
KEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICS
IIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFM
KSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTE
TTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYID
LLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFK
KSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVP
PFYQLCFIPV
|
|
|
|---|
| BDBM50307942 |
|---|
| n/a |
|---|
| Name | BDBM50307942 |
|---|
| Synonyms: | 4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide | CHEMBL598194 | US8796293, 69 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H21ClN6O |
|---|
| Mol. Mass. | 384.863 |
|---|
| SMILES | NC1(CCN(CC1)c1ncnc2[nH]ccc12)C(=O)NCc1ccc(Cl)cc1 |
|---|
| Structure | 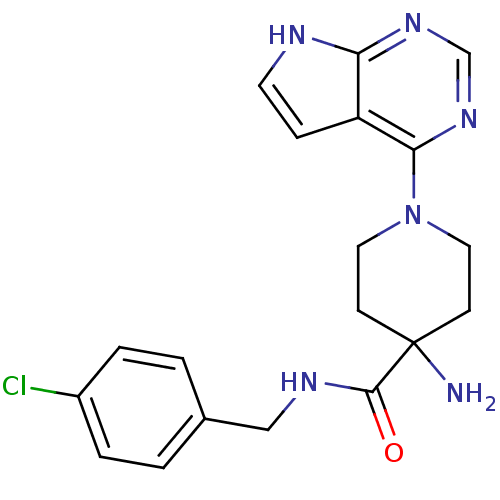
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 McHardy, T; Caldwell, JJ; Cheung, KM; Hunter, LJ; Taylor, K; Rowlands, M; Ruddle, R; Henley, A; de Haven Brandon, A; Valenti, M; Davies, TG; Fazal, L; Seavers, L; Raynaud, FI; Eccles, SA; Aherne, GW; Garrett, MD; Collins, I Discovery of 4-amino-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamides as selective, orally active inhibitors of protein kinase B (Akt). J Med Chem53:2239-49 (2010) [PubMed] Article
McHardy, T; Caldwell, JJ; Cheung, KM; Hunter, LJ; Taylor, K; Rowlands, M; Ruddle, R; Henley, A; de Haven Brandon, A; Valenti, M; Davies, TG; Fazal, L; Seavers, L; Raynaud, FI; Eccles, SA; Aherne, GW; Garrett, MD; Collins, I Discovery of 4-amino-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamides as selective, orally active inhibitors of protein kinase B (Akt). J Med Chem53:2239-49 (2010) [PubMed] Article