| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50312898 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_615895 (CHEMBL1102909) |
|---|
| IC50 | 580±n/a nM |
|---|
| Citation |  Takahashi, J; Hijikuro, I; Kihara, T; Murugesh, MG; Fuse, S; Tsumura, Y; Akaike, A; Niidome, T; Takahashi, T; Sugimoto, H Design, synthesis and evaluation of carbamate-modified (-)-N(1)-phenethylnorphysostigmine derivatives as selective butyrylcholinesterase inhibitors. Bioorg Med Chem Lett20:1721-3 (2010) [PubMed] Article Takahashi, J; Hijikuro, I; Kihara, T; Murugesh, MG; Fuse, S; Tsumura, Y; Akaike, A; Niidome, T; Takahashi, T; Sugimoto, H Design, synthesis and evaluation of carbamate-modified (-)-N(1)-phenethylnorphysostigmine derivatives as selective butyrylcholinesterase inhibitors. Bioorg Med Chem Lett20:1721-3 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | Acylcholine acylhydrolase | Bche | Butyrylcholine esterase | Butyrylcholinesterase | CHLE_MOUSE | Choline esterase II | Pseudocholinesterase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 68465.99 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | Q03311 |
|---|
| Residue: | 603 |
|---|
| Sequence: | MQTQHTKVTQTHFLLWILLLCMPFGKSHTEEDFIITTKTGRVRGLSMPVLGGTVTAFLGI
PYAQPPLGSLRFKKPQPLNKWPDIHNATQYANSCYQNIDQAFPGFQGSEMWNPNTNLSED
CLYLNVWIPVPKPKNATVMVWIYGGGFQTGTSSLPVYDGKFLARVERVIVVSMNYRVGAL
GFLAFPGNPDAPGNMGLFDQQLALQWVQRNIAAFGGNPKSITIFGESAGAASVSLHLLCP
QSYPLFTRAILESGSSNAPWAVKHPEEARNRTLTLAKFTGCSKENEMEMIKCLRSKDPQE
ILRNERFVLPSDSILSINFGPTVDGDFLTDMPHTLLQLGKVKKAQILVGVNKDEGTAFLV
YGAPGFSKDNDSLITRKEFQEGLNMYFPGVSRLGKEAVLFYYVDWLGEQSPEVYRDALDD
VIGDYNIICPALEFTKKFAELENNAFFYFFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLG
RRVNYTRAEEIFSRSIMKTWANFAKYGHPNGTQGNSTMWPVFTSTEQKYLTLNTEKSKIY
SKLRAPQCQFWRLFFPKVLEMTGDIDETEQEWKAGFHRWSNYMMDWQNQFNDYTSKKESC
TAL
|
|
|
|---|
| BDBM50312898 |
|---|
| n/a |
|---|
| Name | BDBM50312898 |
|---|
| Synonyms: | 3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alpha-hexahydropyrrolo[2,3-b]indol-5-ylnaphthalen-2-ylcarbamate | CHEMBL1081455 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H31N3O2 |
|---|
| Mol. Mass. | 477.5967 |
|---|
| SMILES | CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)Nc3ccc4ccccc4c3)ccc12 |r| |
|---|
| Structure | 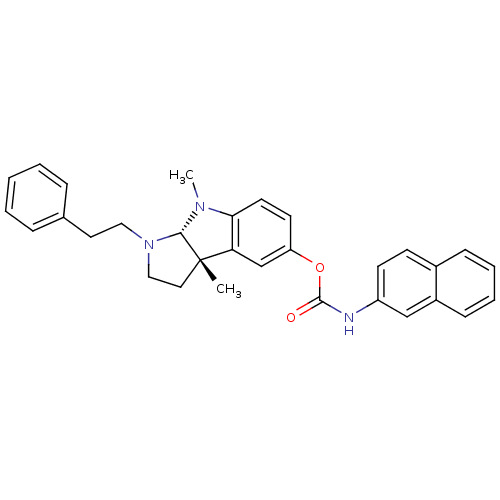
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Takahashi, J; Hijikuro, I; Kihara, T; Murugesh, MG; Fuse, S; Tsumura, Y; Akaike, A; Niidome, T; Takahashi, T; Sugimoto, H Design, synthesis and evaluation of carbamate-modified (-)-N(1)-phenethylnorphysostigmine derivatives as selective butyrylcholinesterase inhibitors. Bioorg Med Chem Lett20:1721-3 (2010) [PubMed] Article
Takahashi, J; Hijikuro, I; Kihara, T; Murugesh, MG; Fuse, S; Tsumura, Y; Akaike, A; Niidome, T; Takahashi, T; Sugimoto, H Design, synthesis and evaluation of carbamate-modified (-)-N(1)-phenethylnorphysostigmine derivatives as selective butyrylcholinesterase inhibitors. Bioorg Med Chem Lett20:1721-3 (2010) [PubMed] Article