 Found 140 hits with Last Name = 'takahashi' and Initial = 'j'
Found 140 hits with Last Name = 'takahashi' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305335

(7-hydroxy-4-((6-methylpyridin-2-ylthio)methyl)-2H-...)Show InChI InChI=1S/C16H13NO3S/c1-10-3-2-4-15(17-10)21-9-11-7-16(19)20-14-8-12(18)5-6-13(11)14/h2-8,18H,9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312905
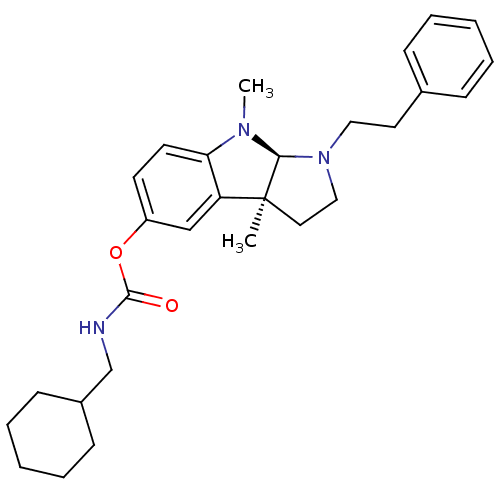
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)NCC3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H37N3O2/c1-28-16-18-31(17-15-21-9-5-3-6-10-21)26(28)30(2)25-14-13-23(19-24(25)28)33-27(32)29-20-22-11-7-4-8-12-22/h3,5-6,9-10,13-14,19,22,26H,4,7-8,11-12,15-18,20H2,1-2H3,(H,29,32)/t26-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305325
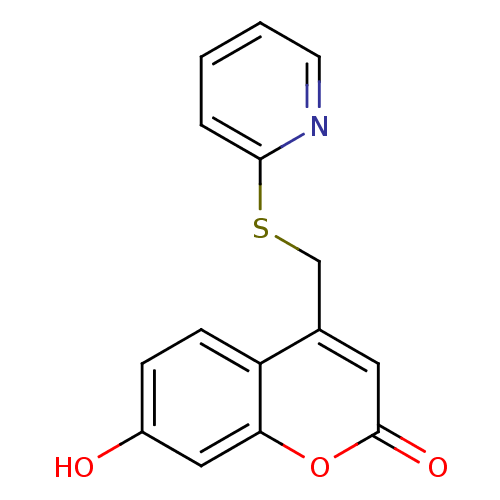
(7-hydroxy-4-((pyridin-2-ylthio)methyl)-2H-chromen-...)Show InChI InChI=1S/C15H11NO3S/c17-11-4-5-12-10(7-15(18)19-13(12)8-11)9-20-14-3-1-2-6-16-14/h1-8,17H,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312902
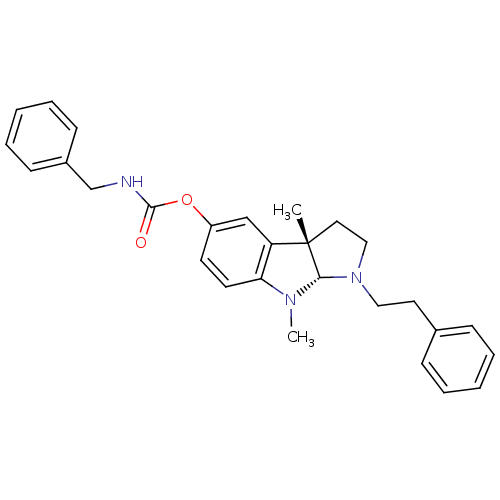
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)NCc3ccccc3)ccc12 |r| Show InChI InChI=1S/C28H31N3O2/c1-28-16-18-31(17-15-21-9-5-3-6-10-21)26(28)30(2)25-14-13-23(19-24(25)28)33-27(32)29-20-22-11-7-4-8-12-22/h3-14,19,26H,15-18,20H2,1-2H3,(H,29,32)/t26-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305336
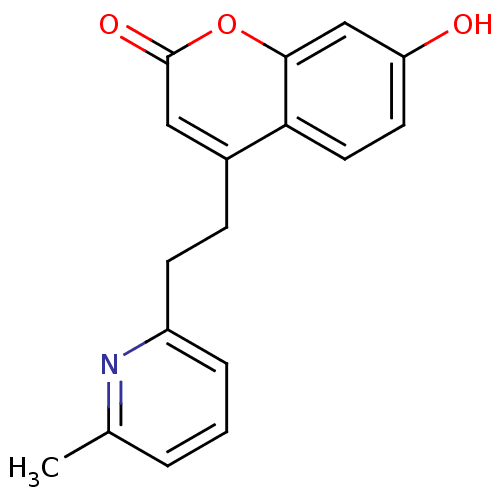
(7-hydroxy-4-(2-(6-methylpyridin-2-yl)ethyl)-2H-chr...)Show InChI InChI=1S/C17H15NO3/c1-11-3-2-4-13(18-11)6-5-12-9-17(20)21-16-10-14(19)7-8-15(12)16/h2-4,7-10,19H,5-6H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50452609

(CHEMBL4206099)Show SMILES CC[C@@H](NC(=O)N1C\C(NC[C@@H](Cc2cc(Cl)ccc2OC)C1=O)=N\Oc1ccccc1)c1ccc(C(O)=O)c(N)c1 |r| Show InChI InChI=1S/C30H32ClN5O6/c1-3-25(18-9-11-23(29(38)39)24(32)15-18)34-30(40)36-17-27(35-42-22-7-5-4-6-8-22)33-16-20(28(36)37)13-19-14-21(31)10-12-26(19)41-2/h4-12,14-15,20,25H,3,13,16-17,32H2,1-2H3,(H,33,35)(H,34,40)(H,38,39)/t20-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... |
Bioorg Med Chem Lett 28: 188-192 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.031
BindingDB Entry DOI: 10.7270/Q2T72M14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305330
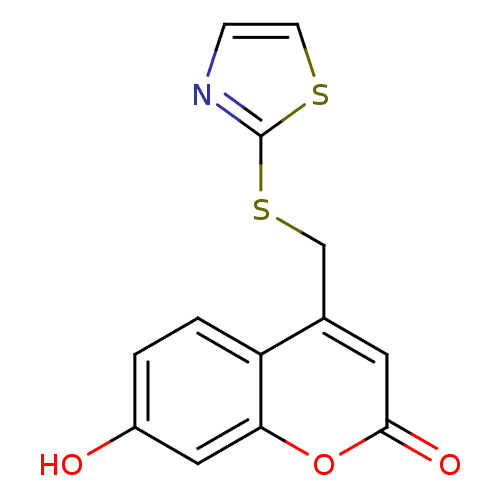
(7-hydroxy-4-((thiazol-2-ylthio)methyl)-2H-chromen-...)Show InChI InChI=1S/C13H9NO3S2/c15-9-1-2-10-8(5-12(16)17-11(10)6-9)7-19-13-14-3-4-18-13/h1-6,15H,7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50014113
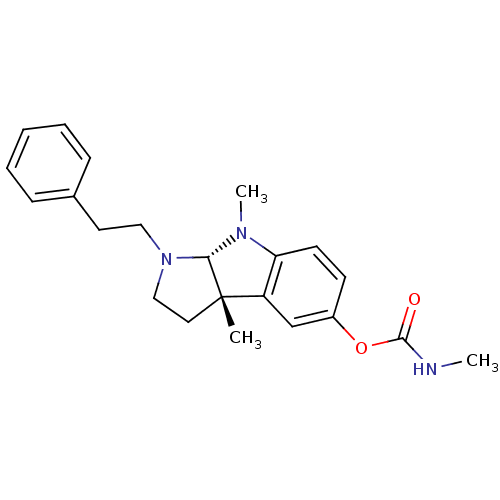
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H27N3O2/c1-22-12-14-25(13-11-16-7-5-4-6-8-16)20(22)24(3)19-10-9-17(15-18(19)22)27-21(26)23-2/h4-10,15,20H,11-14H2,1-3H3,(H,23,26)/t20-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312892
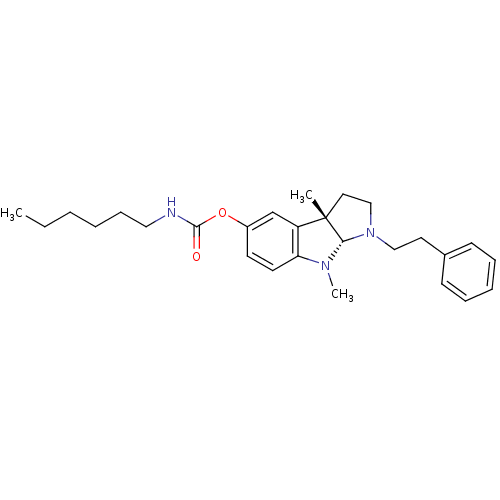
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CCCCCCNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C27H37N3O2/c1-4-5-6-10-17-28-26(31)32-22-13-14-24-23(20-22)27(2)16-19-30(25(27)29(24)3)18-15-21-11-8-7-9-12-21/h7-9,11-14,20,25H,4-6,10,15-19H2,1-3H3,(H,28,31)/t25-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312903
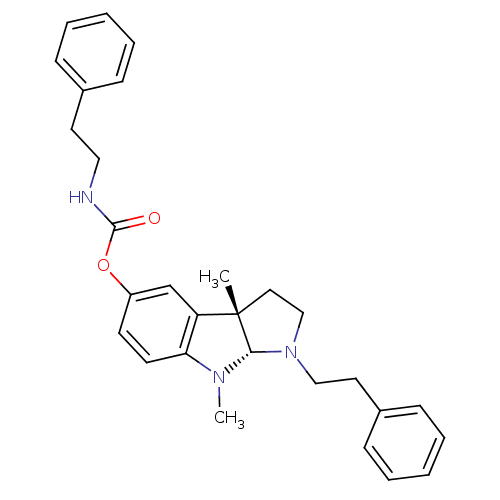
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)NCCc3ccccc3)ccc12 |r| Show InChI InChI=1S/C29H33N3O2/c1-29-17-20-32(19-16-23-11-7-4-8-12-23)27(29)31(2)26-14-13-24(21-25(26)29)34-28(33)30-18-15-22-9-5-3-6-10-22/h3-14,21,27H,15-20H2,1-2H3,(H,30,33)/t27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305320
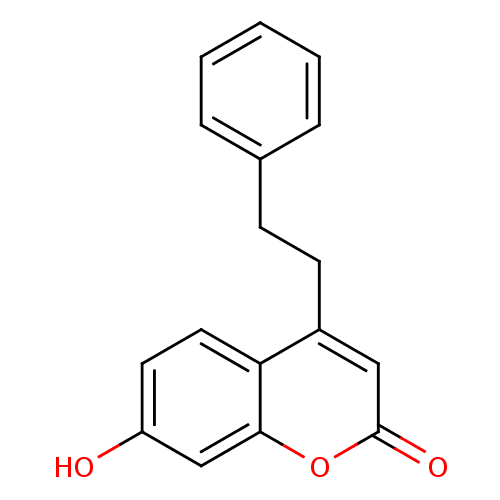
(7-hydroxy-4-phenethyl-2H-chromen-2-one | CHEMBL592...)Show InChI InChI=1S/C17H14O3/c18-14-8-9-15-13(10-17(19)20-16(15)11-14)7-6-12-4-2-1-3-5-12/h1-5,8-11,18H,6-7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312904
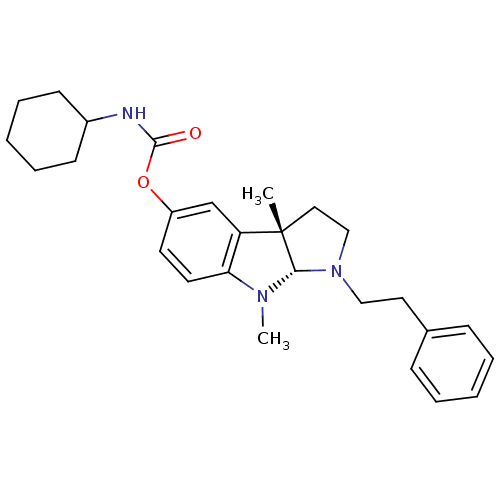
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)NC3CCCCC3)ccc12 |r| Show InChI InChI=1S/C27H35N3O2/c1-27-16-18-30(17-15-20-9-5-3-6-10-20)25(27)29(2)24-14-13-22(19-23(24)27)32-26(31)28-21-11-7-4-8-12-21/h3,5-6,9-10,13-14,19,21,25H,4,7-8,11-12,15-18H2,1-2H3,(H,28,31)/t25-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305338
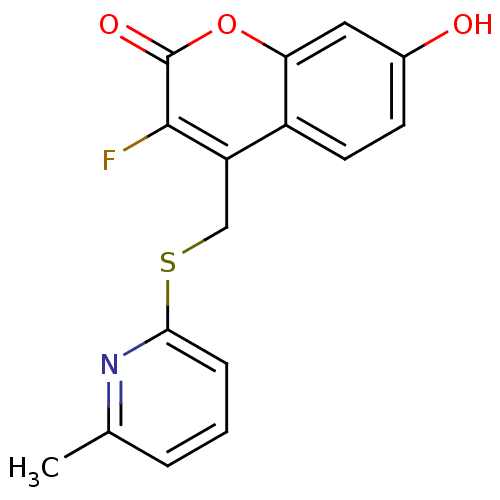
(3-fluoro-7-hydroxy-4-((6-methylpyridin-2-ylthio)me...)Show InChI InChI=1S/C16H12FNO3S/c1-9-3-2-4-14(18-9)22-8-12-11-6-5-10(19)7-13(11)21-16(20)15(12)17/h2-7,19H,8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305316
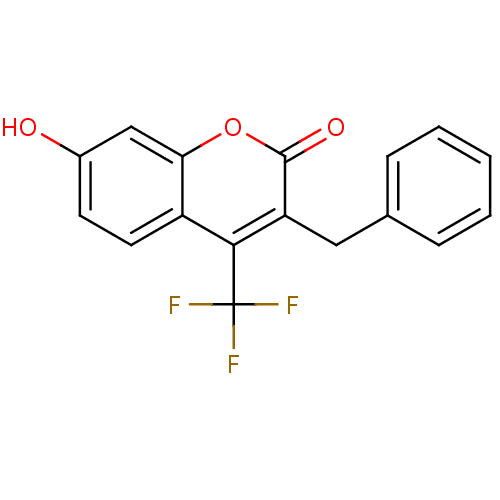
(3-benzyl-7-hydroxy-4-(trifluoromethyl)-2H-chromen-...)Show InChI InChI=1S/C17H11F3O3/c18-17(19,20)15-12-7-6-11(21)9-14(12)23-16(22)13(15)8-10-4-2-1-3-5-10/h1-7,9,21H,8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1718-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.057
BindingDB Entry DOI: 10.7270/Q2RV0NV4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305339
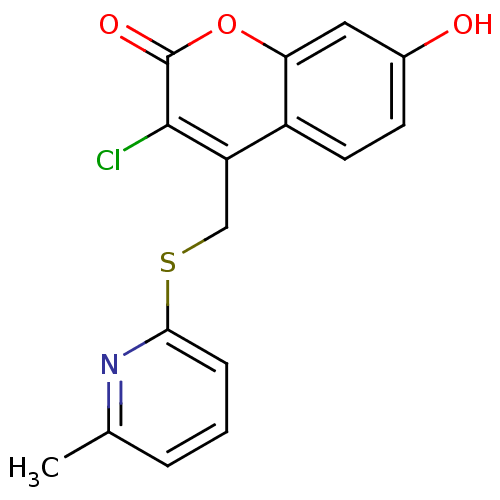
(3-chloro-7-hydroxy-4-((6-methylpyridin-2-ylthio)me...)Show InChI InChI=1S/C16H12ClNO3S/c1-9-3-2-4-14(18-9)22-8-12-11-6-5-10(19)7-13(11)21-16(20)15(12)17/h2-7,19H,8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50452608
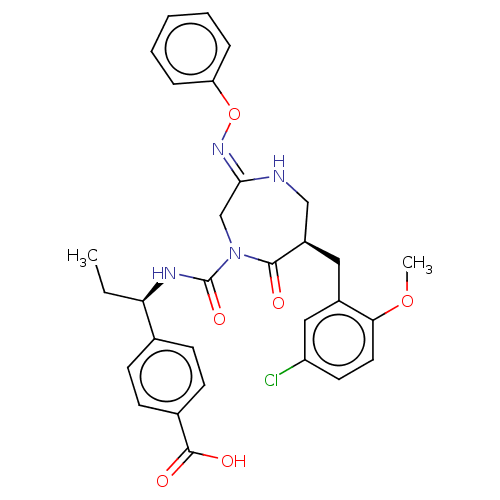
(CHEMBL4210939)Show SMILES CC[C@@H](NC(=O)N1C\C(NC[C@@H](Cc2cc(Cl)ccc2OC)C1=O)=N\Oc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C30H31ClN4O6/c1-3-25(19-9-11-20(12-10-19)29(37)38)33-30(39)35-18-27(34-41-24-7-5-4-6-8-24)32-17-22(28(35)36)15-21-16-23(31)13-14-26(21)40-2/h4-14,16,22,25H,3,15,17-18H2,1-2H3,(H,32,34)(H,33,39)(H,37,38)/t22-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... |
Bioorg Med Chem Lett 28: 188-192 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.031
BindingDB Entry DOI: 10.7270/Q2T72M14 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305309
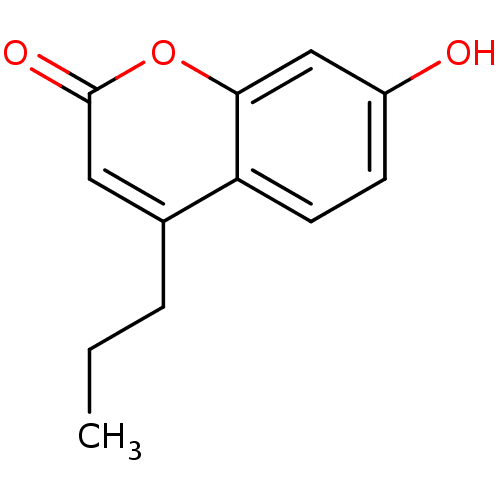
(7-hydroxy-4-propyl-2H-chromen-2-one | CHEMBL605407)Show InChI InChI=1S/C12H12O3/c1-2-3-8-6-12(14)15-11-7-9(13)4-5-10(8)11/h4-7,13H,2-3H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50452612
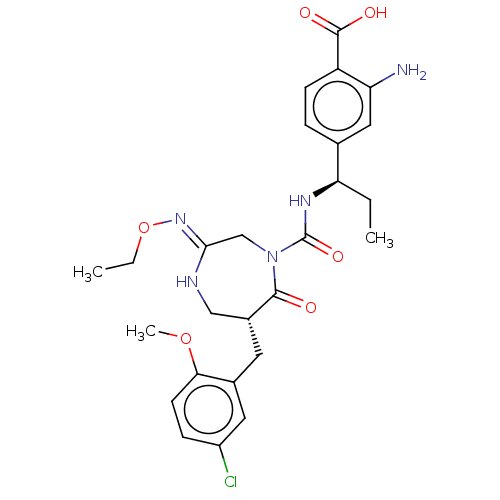
(CHEMBL4202858)Show SMILES CCO\N=C1\CN(C(=O)N[C@H](CC)c2ccc(C(O)=O)c(N)c2)C(=O)[C@H](Cc2cc(Cl)ccc2OC)CN1 |r| Show InChI InChI=1S/C26H32ClN5O6/c1-4-21(15-6-8-19(25(34)35)20(28)12-15)30-26(36)32-14-23(31-38-5-2)29-13-17(24(32)33)10-16-11-18(27)7-9-22(16)37-3/h6-9,11-12,17,21H,4-5,10,13-14,28H2,1-3H3,(H,29,31)(H,30,36)(H,34,35)/t17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... |
Bioorg Med Chem Lett 28: 188-192 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.031
BindingDB Entry DOI: 10.7270/Q2T72M14 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312897
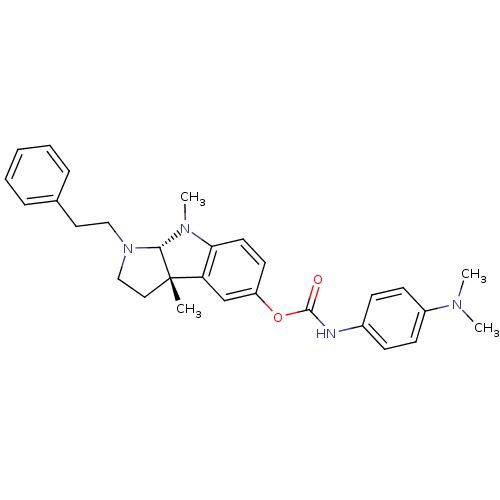
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN(C)c1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5ccccc5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C29H34N4O2/c1-29-17-19-33(18-16-21-8-6-5-7-9-21)27(29)32(4)26-15-14-24(20-25(26)29)35-28(34)30-22-10-12-23(13-11-22)31(2)3/h5-15,20,27H,16-19H2,1-4H3,(H,30,34)/t27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312887
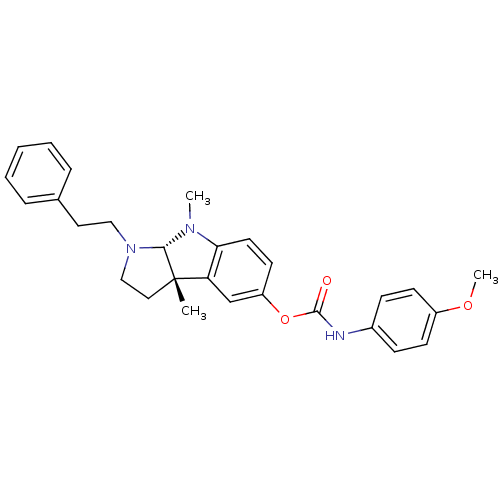
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5ccccc5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C28H31N3O3/c1-28-16-18-31(17-15-20-7-5-4-6-8-20)26(28)30(2)25-14-13-23(19-24(25)28)34-27(32)29-21-9-11-22(33-3)12-10-21/h4-14,19,26H,15-18H2,1-3H3,(H,29,32)/t26-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305333
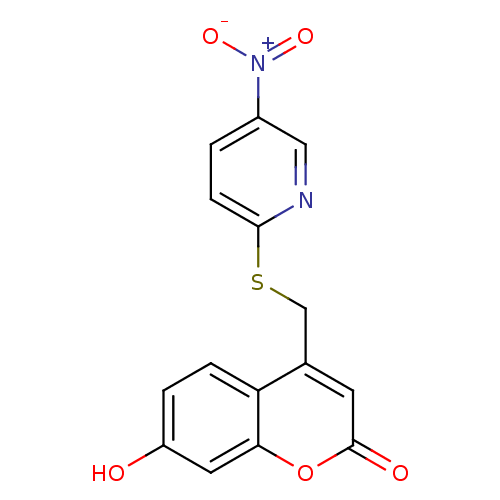
(7-hydroxy-4-((4-nitropyridin-2-ylthio)methyl)-2H-c...)Show SMILES Oc1ccc2c(CSc3ccc(cn3)[N+]([O-])=O)cc(=O)oc2c1 Show InChI InChI=1S/C15H10N2O5S/c18-11-2-3-12-9(5-15(19)22-13(12)6-11)8-23-14-4-1-10(7-16-14)17(20)21/h1-7,18H,8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305332
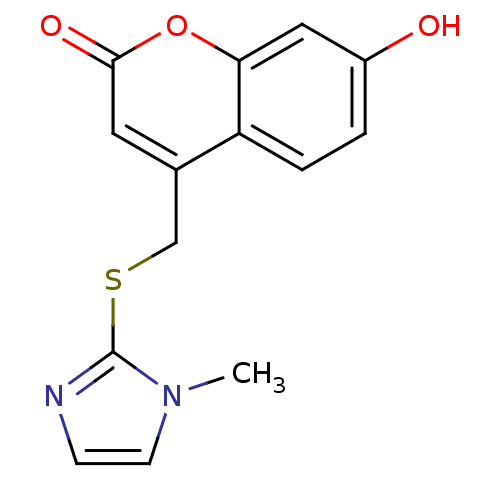
(7-hydroxy-4-((1-methyl-1H-imidazol-2-ylthio)methyl...)Show InChI InChI=1S/C14H12N2O3S/c1-16-5-4-15-14(16)20-8-9-6-13(18)19-12-7-10(17)2-3-11(9)12/h2-7,17H,8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305322
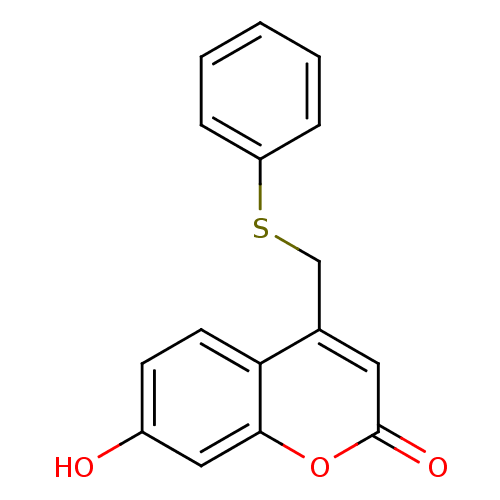
(7-hydroxy-4-(phenylthiomethyl)-2H-chromen-2-one | ...)Show InChI InChI=1S/C16H12O3S/c17-12-6-7-14-11(8-16(18)19-15(14)9-12)10-20-13-4-2-1-3-5-13/h1-9,17H,10H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305331
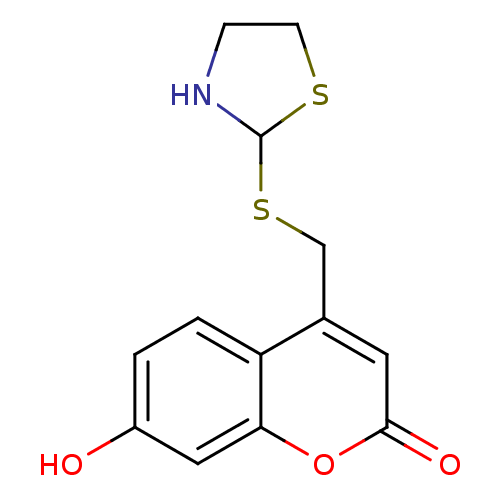
(7-hydroxy-4-((thiazolidin-2-ylthio)methyl)-2H-chro...)Show InChI InChI=1S/C13H13NO3S2/c15-9-1-2-10-8(5-12(16)17-11(10)6-9)7-19-13-14-3-4-18-13/h1-2,5-6,13-15H,3-4,7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312890
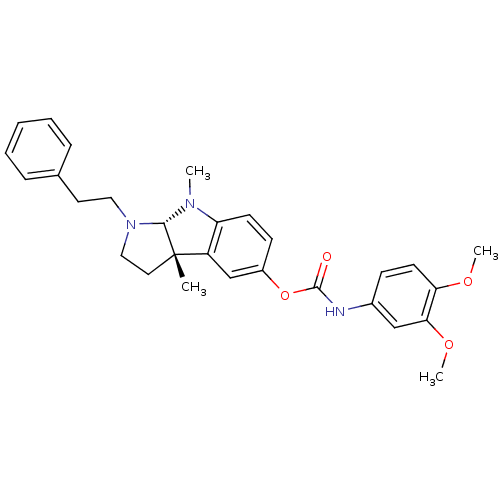
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5ccccc5)CC[C@@]4(C)c3c2)cc1OC |r| Show InChI InChI=1S/C29H33N3O4/c1-29-15-17-32(16-14-20-8-6-5-7-9-20)27(29)31(2)24-12-11-22(19-23(24)29)36-28(33)30-21-10-13-25(34-3)26(18-21)35-4/h5-13,18-19,27H,14-17H2,1-4H3,(H,30,33)/t27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305318

(3-Chloro-4-methyl-7-hydroxy-2H-2-chromenone | 3-ch...)Show InChI InChI=1S/C10H7ClO3/c1-5-7-3-2-6(12)4-8(7)14-10(13)9(5)11/h2-4,12H,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312891
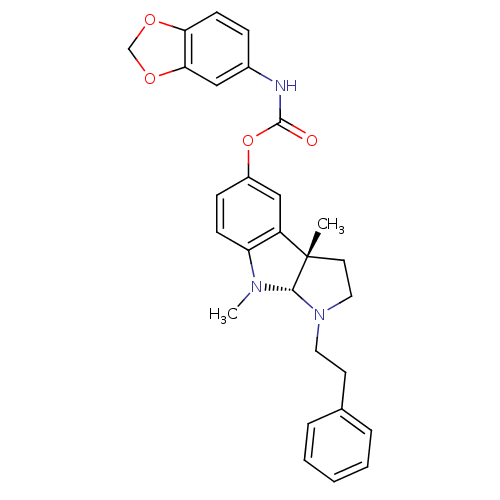
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)Nc3ccc4OCOc4c3)ccc12 |r| Show InChI InChI=1S/C28H29N3O4/c1-28-13-15-31(14-12-19-6-4-3-5-7-19)26(28)30(2)23-10-9-21(17-22(23)28)35-27(32)29-20-8-11-24-25(16-20)34-18-33-24/h3-11,16-17,26H,12-15,18H2,1-2H3,(H,29,32)/t26-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305308
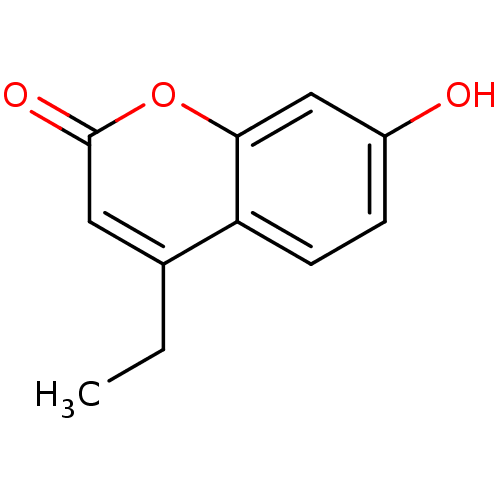
(4-ethyl-7-hydroxy-2H-chromen-2-one | CHEMBL600114)Show InChI InChI=1S/C11H10O3/c1-2-7-5-11(13)14-10-6-8(12)3-4-9(7)10/h3-6,12H,2H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312815

((3aS,8aR)-3a,8-dimethyl-1-(2-(pyridin-2-yl)ethyl)-...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5ccccn5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C29H34N4O2/c1-20(2)21-8-10-23(11-9-21)31-28(34)35-24-12-13-26-25(19-24)29(3)15-18-33(27(29)32(26)4)17-14-22-7-5-6-16-30-22/h5-13,16,19-20,27H,14-15,17-18H2,1-4H3,(H,31,34)/t27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1718-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.057
BindingDB Entry DOI: 10.7270/Q2RV0NV4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312818
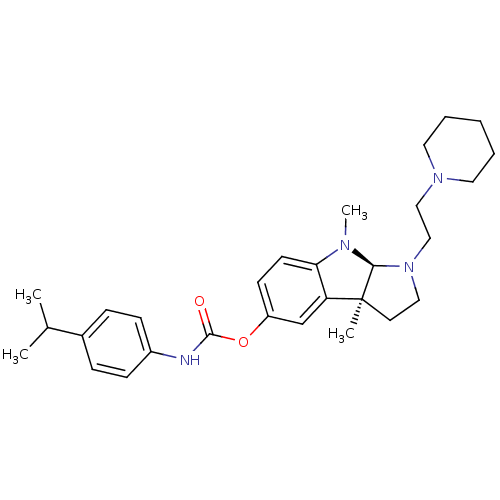
((3aS,8aR)-3a,8-dimethyl-1-(2-(piperidin-1-yl)ethyl...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCN5CCCCC5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C29H40N4O2/c1-21(2)22-8-10-23(11-9-22)30-28(34)35-24-12-13-26-25(20-24)29(3)14-17-33(27(29)31(26)4)19-18-32-15-6-5-7-16-32/h8-13,20-21,27H,5-7,14-19H2,1-4H3,(H,30,34)/t27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1718-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.057
BindingDB Entry DOI: 10.7270/Q2RV0NV4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312817
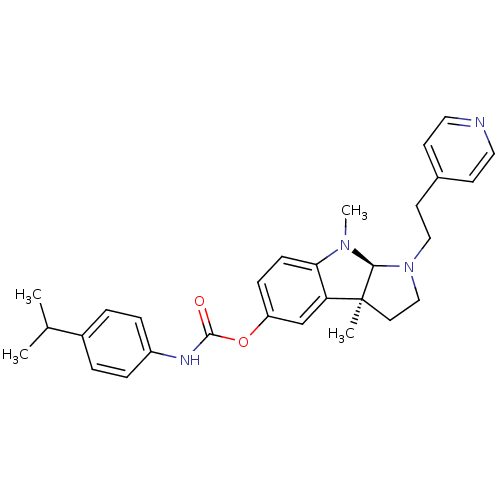
((3aS,8aR)-3a,8-dimethyl-1-(2-(pyridin-4-yl)ethyl)-...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5ccncc5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C29H34N4O2/c1-20(2)22-5-7-23(8-6-22)31-28(34)35-24-9-10-26-25(19-24)29(3)14-18-33(27(29)32(26)4)17-13-21-11-15-30-16-12-21/h5-12,15-16,19-20,27H,13-14,17-18H2,1-4H3,(H,31,34)/t27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1718-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.057
BindingDB Entry DOI: 10.7270/Q2RV0NV4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50014113

(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H27N3O2/c1-22-12-14-25(13-11-16-7-5-4-6-8-16)20(22)24(3)19-10-9-17(15-18(19)22)27-21(26)23-2/h4-10,15,20H,11-14H2,1-3H3,(H,23,26)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50312902

(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)NCc3ccccc3)ccc12 |r| Show InChI InChI=1S/C28H31N3O2/c1-28-16-18-31(17-15-21-9-5-3-6-10-21)26(28)30(2)25-14-13-23(19-24(25)28)33-27(32)29-20-22-11-7-4-8-12-22/h3-14,19,26H,15-18,20H2,1-2H3,(H,29,32)/t26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312816
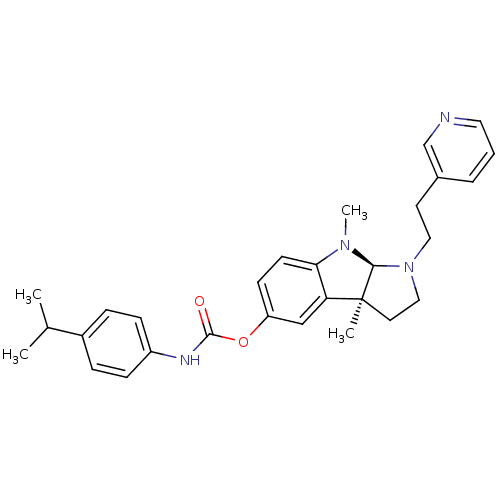
((3aS,8aR)-3a,8-dimethyl-1-(2-(pyridin-3-yl)ethyl)-...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(CCc5cccnc5)CC[C@@]4(C)c3c2)cc1 |r| Show InChI InChI=1S/C29H34N4O2/c1-20(2)22-7-9-23(10-8-22)31-28(34)35-24-11-12-26-25(18-24)29(3)14-17-33(27(29)32(26)4)16-13-21-6-5-15-30-19-21/h5-12,15,18-20,27H,13-14,16-17H2,1-4H3,(H,31,34)/t27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1718-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.057
BindingDB Entry DOI: 10.7270/Q2RV0NV4 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305310
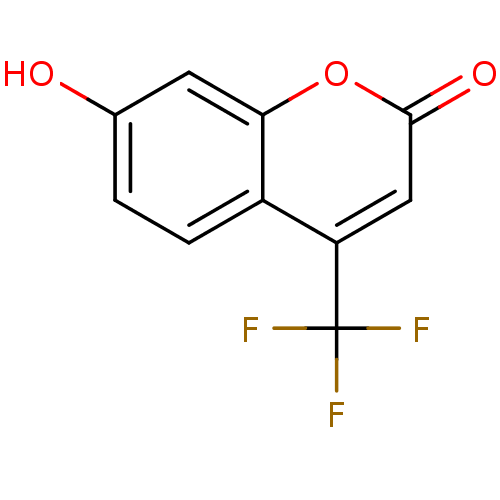
(7-Hydroxy-4-trifluoromethyl-chromen-2-one | 7-hydr...)Show InChI InChI=1S/C10H5F3O3/c11-10(12,13)7-4-9(15)16-8-3-5(14)1-2-6(7)8/h1-4,14H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305315
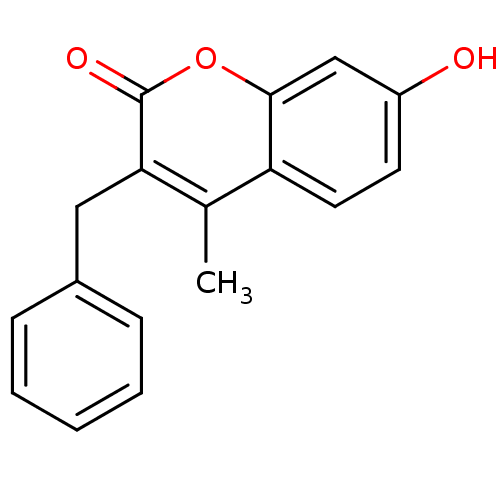
(3-benzyl-7-hydroxy-4-methyl-2H-chromen-2-one | CHE...)Show InChI InChI=1S/C17H14O3/c1-11-14-8-7-13(18)10-16(14)20-17(19)15(11)9-12-5-3-2-4-6-12/h2-8,10,18H,9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305329
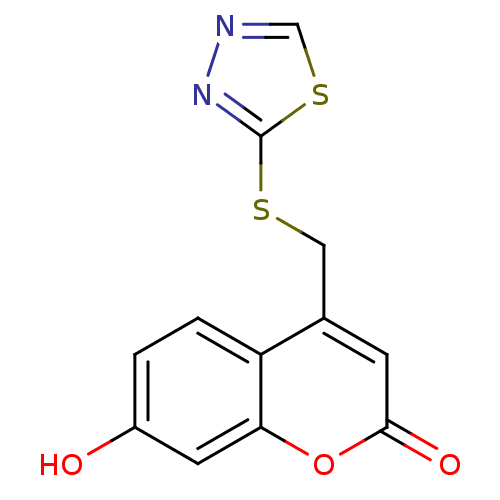
(4-((1,3,4-thiadiazol-2-ylthio)methyl)-7-hydroxy-2H...)Show InChI InChI=1S/C12H8N2O3S2/c15-8-1-2-9-7(3-11(16)17-10(9)4-8)5-18-12-14-13-6-19-12/h1-4,6,15H,5H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50282524
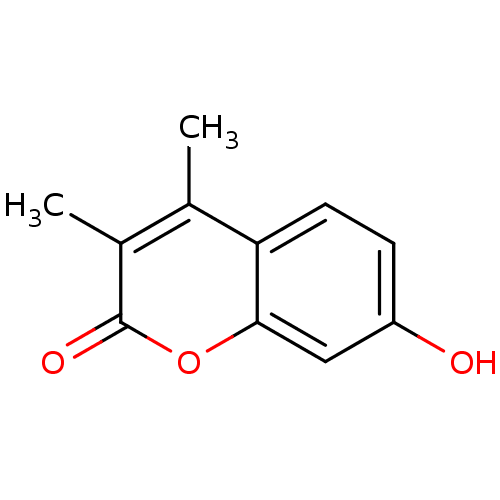
(7-Hydroxy-3,4-dimethyl-chromen-2-one | 7-hydroxy-3...)Show InChI InChI=1S/C11H10O3/c1-6-7(2)11(13)14-10-5-8(12)3-4-9(6)10/h3-5,12H,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305334
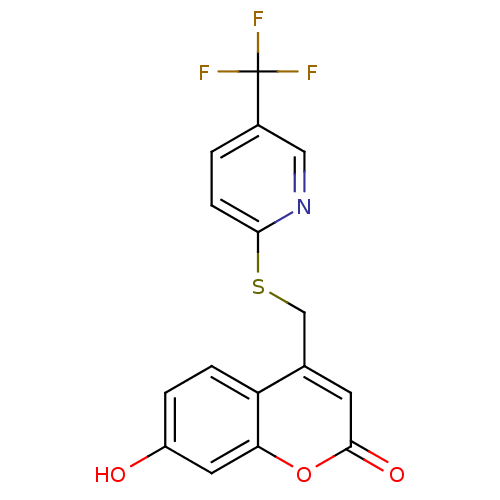
(7-hydroxy-4-((5-(trifluoromethyl)pyridin-2-ylthio)...)Show InChI InChI=1S/C16H10F3NO3S/c17-16(18,19)10-1-4-14(20-7-10)24-8-9-5-15(22)23-13-6-11(21)2-3-12(9)13/h1-7,21H,8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305314

(7-hydroxy-4-methyl-3-propyl-2H-chromen-2-one | CHE...)Show InChI InChI=1S/C13H14O3/c1-3-4-11-8(2)10-6-5-9(14)7-12(10)16-13(11)15/h5-7,14H,3-4H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50452605
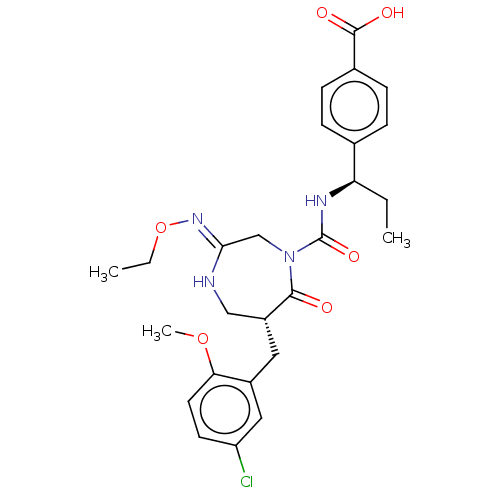
(CHEMBL4216202)Show SMILES CCO\N=C1\CN(C(=O)N[C@H](CC)c2ccc(cc2)C(O)=O)C(=O)[C@H](Cc2cc(Cl)ccc2OC)CN1 |r| Show InChI InChI=1S/C26H31ClN4O6/c1-4-21(16-6-8-17(9-7-16)25(33)34)29-26(35)31-15-23(30-37-5-2)28-14-19(24(31)32)12-18-13-20(27)10-11-22(18)36-3/h6-11,13,19,21H,4-5,12,14-15H2,1-3H3,(H,28,30)(H,29,35)(H,33,34)/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... |
Bioorg Med Chem Lett 28: 188-192 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.031
BindingDB Entry DOI: 10.7270/Q2T72M14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Mus musculus (Mouse)) | BDBM10960
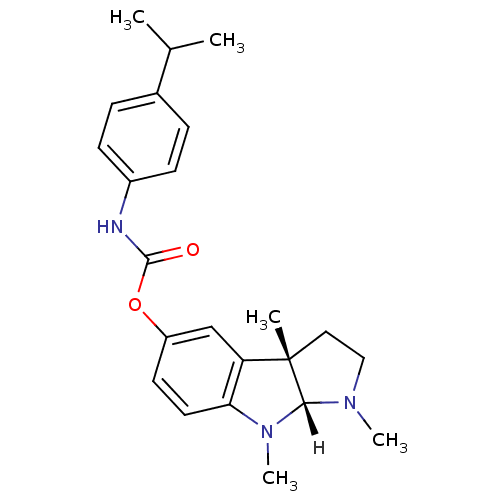
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccc(cc3)C(C)C)ccc1N2C |r| Show InChI InChI=1S/C23H29N3O2/c1-15(2)16-6-8-17(9-7-16)24-22(27)28-18-10-11-20-19(14-18)23(3)12-13-25(4)21(23)26(20)5/h6-11,14-15,21H,12-13H2,1-5H3,(H,24,27)/t21-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1718-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.057
BindingDB Entry DOI: 10.7270/Q2RV0NV4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1718-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.057
BindingDB Entry DOI: 10.7270/Q2RV0NV4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50210730
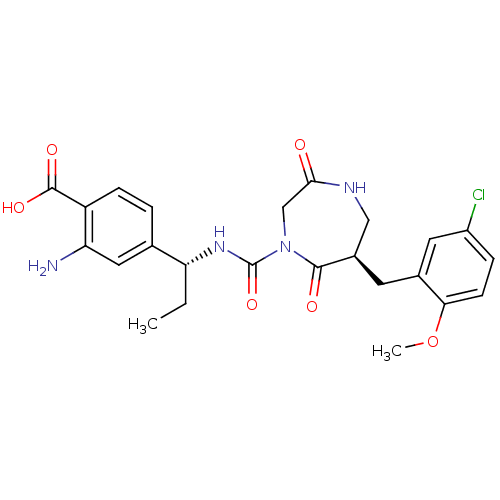
(4-((R)-1-((R)-6-(5-chloro-2-methoxybenzyl)-2,5-dio...)Show SMILES CC[C@@H](NC(=O)N1CC(=O)NC[C@@H](Cc2cc(Cl)ccc2OC)C1=O)c1ccc(C(O)=O)c(N)c1 Show InChI InChI=1S/C24H27ClN4O6/c1-3-19(13-4-6-17(23(32)33)18(26)10-13)28-24(34)29-12-21(30)27-11-15(22(29)31)8-14-9-16(25)5-7-20(14)35-2/h4-7,9-10,15,19H,3,8,11-12,26H2,1-2H3,(H,27,30)(H,28,34)(H,32,33)/t15-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... |
Bioorg Med Chem Lett 28: 188-192 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.031
BindingDB Entry DOI: 10.7270/Q2T72M14 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305340
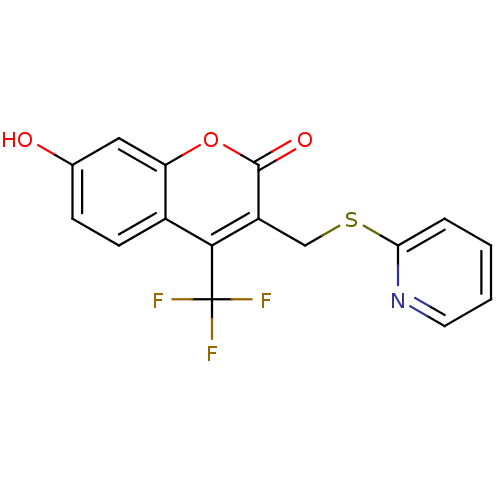
(7-hydroxy-3-((pyridin-2-ylthio)methyl)-4-(trifluor...)Show InChI InChI=1S/C16H10F3NO3S/c17-16(18,19)14-10-5-4-9(21)7-12(10)23-15(22)11(14)8-24-13-3-1-2-6-20-13/h1-7,21H,8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312893
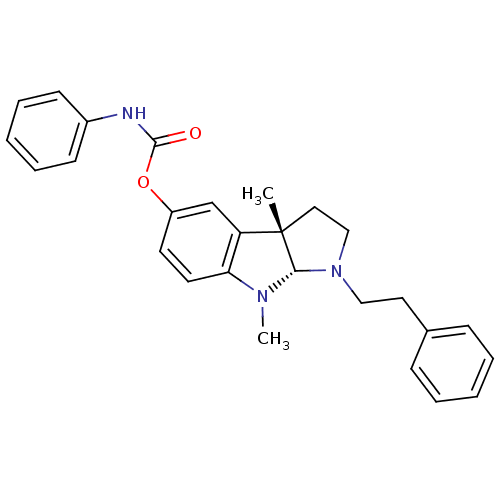
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)Nc3ccccc3)ccc12 |r| Show InChI InChI=1S/C27H29N3O2/c1-27-16-18-30(17-15-20-9-5-3-6-10-20)25(27)29(2)24-14-13-22(19-23(24)27)32-26(31)28-21-11-7-4-8-12-21/h3-14,19,25H,15-18H2,1-2H3,(H,28,31)/t25-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50312899
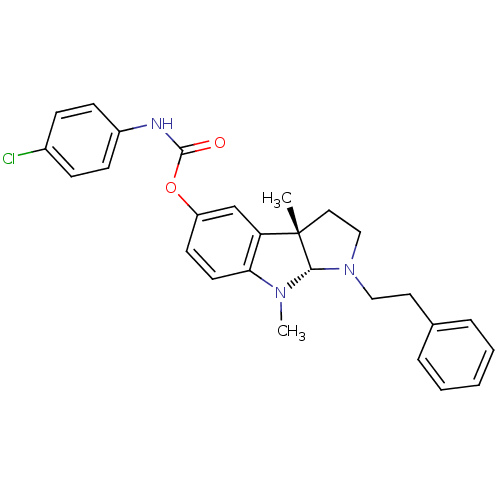
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CN1[C@H]2N(CCc3ccccc3)CC[C@@]2(C)c2cc(OC(=O)Nc3ccc(Cl)cc3)ccc12 |r| Show InChI InChI=1S/C27H28ClN3O2/c1-27-15-17-31(16-14-19-6-4-3-5-7-19)25(27)30(2)24-13-12-22(18-23(24)27)33-26(32)29-21-10-8-20(28)9-11-21/h3-13,18,25H,14-17H2,1-2H3,(H,29,32)/t25-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE after 1 hr by modified Ellman's colorimetric method |
Bioorg Med Chem Lett 20: 1721-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.035
BindingDB Entry DOI: 10.7270/Q27S7NWW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data