| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Bifunctional epoxide hydrolase 2 |
|---|
| Ligand | BDBM50305629 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_635052 (CHEMBL1118603) |
|---|
| IC50 | 18±n/a nM |
|---|
| Citation |  Kowalski, JA; Swinamer, AD; Muegge, I; Eldrup, AB; Kukulka, A; Cywin, CL; De Lombaert, S Rapid synthesis of an array of trisubstituted urea-based soluble epoxide hydrolase inhibitors facilitated by a novel solid-phase method. Bioorg Med Chem Lett20:3703-7 (2010) [PubMed] Article Kowalski, JA; Swinamer, AD; Muegge, I; Eldrup, AB; Kukulka, A; Cywin, CL; De Lombaert, S Rapid synthesis of an array of trisubstituted urea-based soluble epoxide hydrolase inhibitors facilitated by a novel solid-phase method. Bioorg Med Chem Lett20:3703-7 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Bifunctional epoxide hydrolase 2 |
|---|
| Name: | Bifunctional epoxide hydrolase 2 |
|---|
| Synonyms: | Cytosolic epoxide hydrolase 2 | EBifunctional epoxide hydrolase 2 | EPHX2 | Epoxide hydratase | HYES_HUMAN | Lipid-phosphate phosphatase | Soluble epoxide hydrolase (sEH) | epoxide hydrolase 2, cytoplasmic |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 62613.07 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P34913 |
|---|
| Residue: | 555 |
|---|
| Sequence: | MTLRAAVFDLDGVLALPAVFGVLGRTEEALALPRGLLNDAFQKGGPEGATTRLMKGEITL
SQWIPLMEENCRKCSETAKVCLPKNFSIKEIFDKAISARKINRPMLQAALMLRKKGFTTA
ILTNTWLDDRAERDGLAQLMCELKMHFDFLIESCQVGMVKPEPQIYKFLLDTLKASPSEV
VFLDDIGANLKPARDLGMVTILVQDTDTALKELEKVTGIQLLNTPAPLPTSCNPSDMSHG
YVTVKPRVRLHFVELGSGPAVCLCHGFPESWYSWRYQIPALAQAGYRVLAMDMKGYGESS
APPEIEEYCMEVLCKEMVTFLDKLGLSQAVFIGHDWGGMLVWYMALFYPERVRAVASLNT
PFIPANPNMSPLESIKANPVFDYQLYFQEPGVAEAELEQNLSRTFKSLFRASDESVLSMH
KVCEAGGLFVNSPEEPSLSRMVTEEEIQFYVQQFKKSGFRGPLNWYRNMERNWKWACKSL
GRKILIPALMVTAEKDFVLVPQMSQHMEDWIPHLKRGHIEDCGHWTQMDKPTEVNQILIK
WLDSDARNPPVVSKM
|
|
|
|---|
| BDBM50305629 |
|---|
| n/a |
|---|
| Name | BDBM50305629 |
|---|
| Synonyms: | CHEMBL589136 | N-(2,4-dichlorobenzyl)-4-(pyrimidin-2-yloxy)piperidine-1-carboxamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H18Cl2N4O2 |
|---|
| Mol. Mass. | 381.256 |
|---|
| SMILES | Clc1ccc(CNC(=O)N2CCC(CC2)Oc2ncccn2)c(Cl)c1 |
|---|
| Structure | 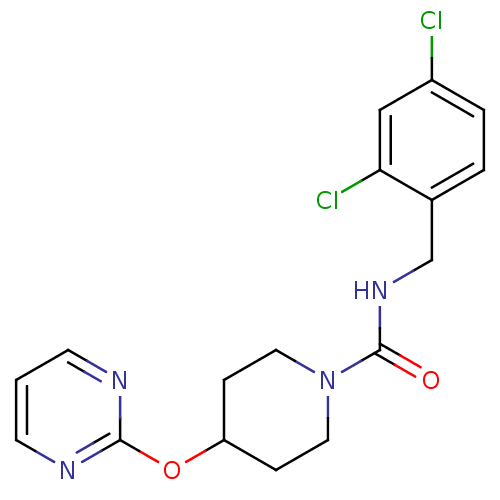
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kowalski, JA; Swinamer, AD; Muegge, I; Eldrup, AB; Kukulka, A; Cywin, CL; De Lombaert, S Rapid synthesis of an array of trisubstituted urea-based soluble epoxide hydrolase inhibitors facilitated by a novel solid-phase method. Bioorg Med Chem Lett20:3703-7 (2010) [PubMed] Article
Kowalski, JA; Swinamer, AD; Muegge, I; Eldrup, AB; Kukulka, A; Cywin, CL; De Lombaert, S Rapid synthesis of an array of trisubstituted urea-based soluble epoxide hydrolase inhibitors facilitated by a novel solid-phase method. Bioorg Med Chem Lett20:3703-7 (2010) [PubMed] Article