

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50302462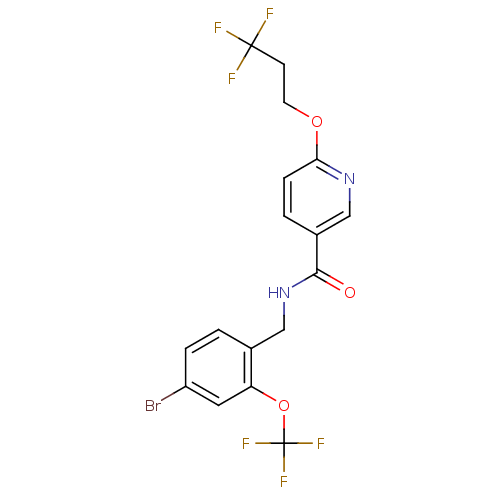 (CHEMBL566648 | N-(4-bromo-2-(trifluoromethoxy)benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of soluble EH in human HepG2 cells by cellular assay | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361618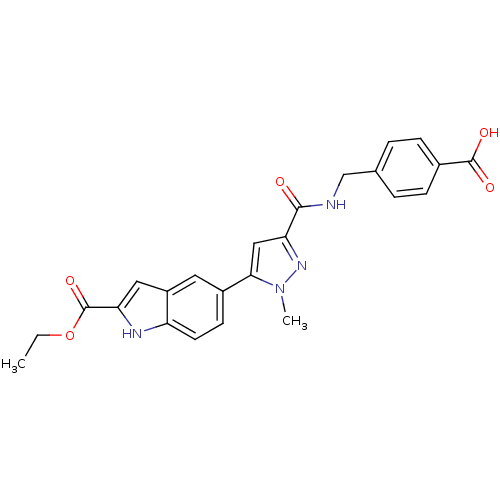 (CHEMBL1939876 | US8785489, 4-{[({5-[2-(ethoxycarbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human MMP13 expressed in Escherichia coli after 30 mins by fluorimetry | J Med Chem 54: 8174-87 (2011) Article DOI: 10.1021/jm201129m BindingDB Entry DOI: 10.7270/Q2C53M9C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50319985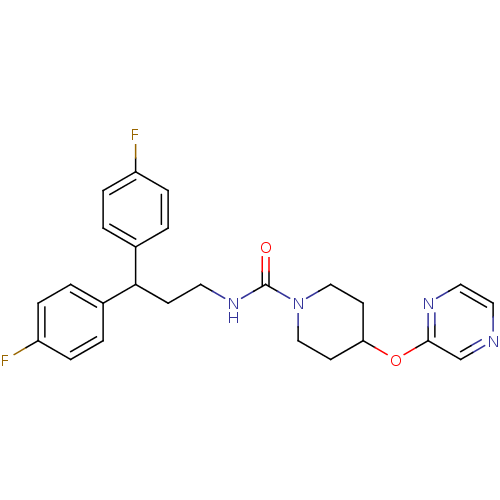 (CHEMBL1083622 | N-(3,3-bis(4-fluorophenyl)propyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase | Bioorg Med Chem Lett 20: 3703-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.078 BindingDB Entry DOI: 10.7270/Q2BV7GTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center Curated by ChEMBL | Assay Description Binding to Estrogen receptor- beta (ER beta) receptor | Bioorg Med Chem Lett 12: 2875-8 (2002) BindingDB Entry DOI: 10.7270/Q2F18Z2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (RAT) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center Curated by ChEMBL | Assay Description Binding to Estrogen receptor- alpha (ER alpha) receptor | Bioorg Med Chem Lett 12: 2875-8 (2002) BindingDB Entry DOI: 10.7270/Q2F18Z2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50302475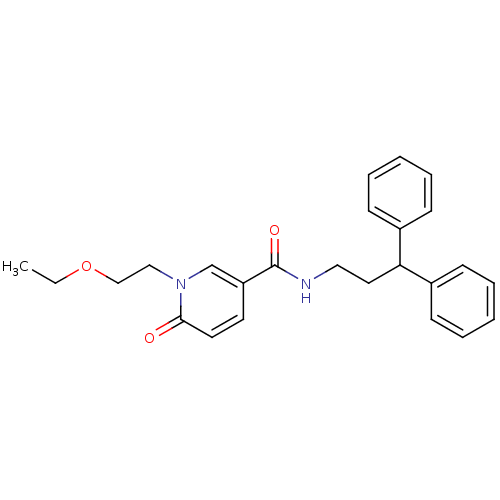 (CHEMBL567491 | N-(3,3-diphenylpropyl)-1-(2-ethoxye...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble EH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361634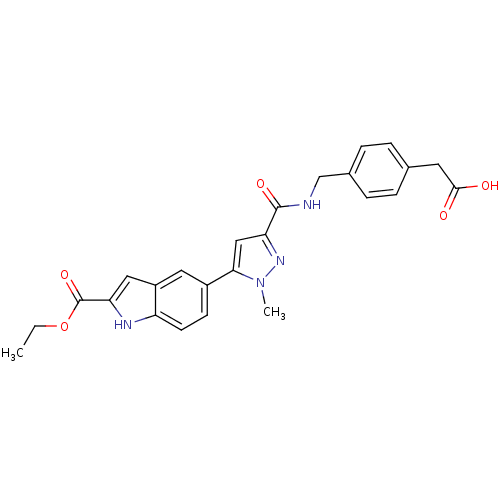 (CHEMBL1939877 | US8785489, (4-{[({5-[2-(ethoxycarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human MMP13 expressed in Escherichia coli after 30 mins by fluorimetry | J Med Chem 54: 8174-87 (2011) Article DOI: 10.1021/jm201129m BindingDB Entry DOI: 10.7270/Q2C53M9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50319986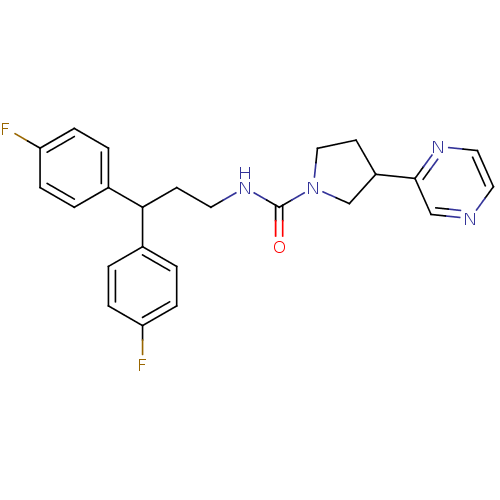 (CHEMBL1085743 | N-(3,3-bis(4-fluorophenyl)propyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase | Bioorg Med Chem Lett 20: 3703-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.078 BindingDB Entry DOI: 10.7270/Q2BV7GTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302475 (CHEMBL567491 | N-(3,3-diphenylpropyl)-1-(2-ethoxye...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50319984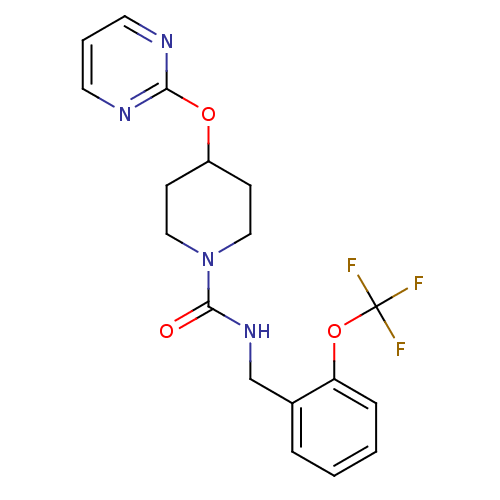 (4-(pyrimidin-2-yloxy)-N-(2-(trifluoromethoxy)benzy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase | Bioorg Med Chem Lett 20: 3703-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.078 BindingDB Entry DOI: 10.7270/Q2BV7GTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297414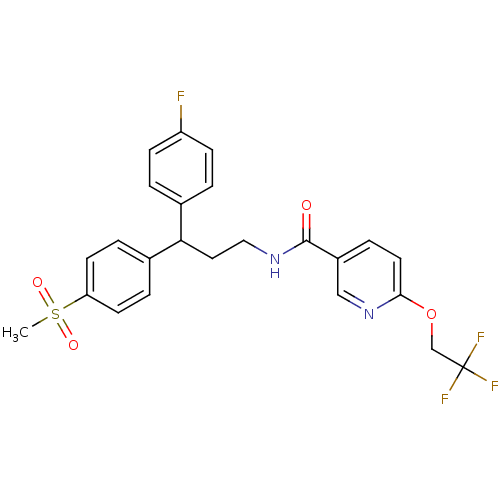 (CHEMBL556234 | N-[3-(4-Fluorophenyl)-3-(4-methanes...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361618 (CHEMBL1939876 | US8785489, 4-{[({5-[2-(ethoxycarbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of MMP13 after 30 mins by fluorimetry in the presence of 1.25% human serum albumin | J Med Chem 54: 8174-87 (2011) Article DOI: 10.1021/jm201129m BindingDB Entry DOI: 10.7270/Q2C53M9C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50319986 (CHEMBL1085743 | N-(3,3-bis(4-fluorophenyl)propyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assay | Bioorg Med Chem Lett 20: 3703-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.078 BindingDB Entry DOI: 10.7270/Q2BV7GTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302461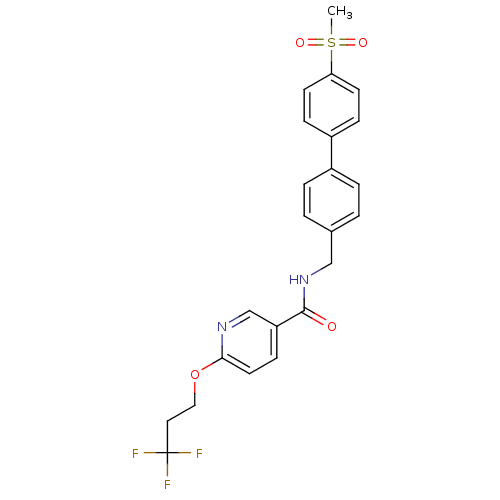 (CHEMBL567703 | N-((4'-(methylsulfonyl)biphenyl-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50302464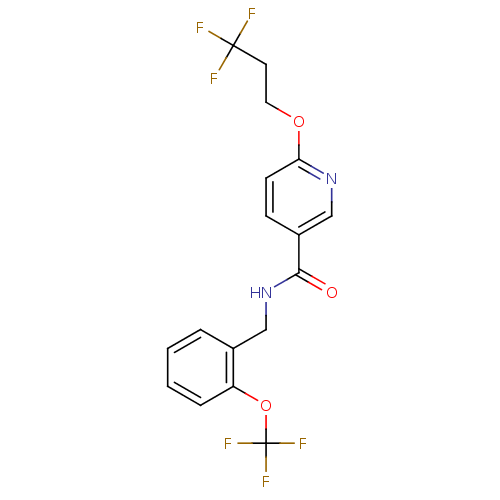 (CHEMBL567110 | N-(2-(trifluoromethoxy)benzyl)-6-(3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble EH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297413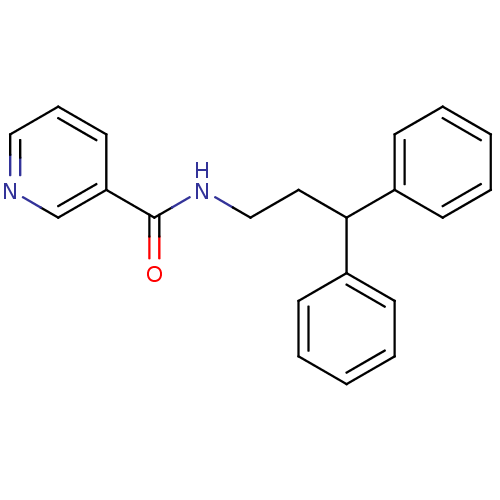 (CHEMBL560537 | N-(3,3-diphenyl-propyl)-nicotinamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297415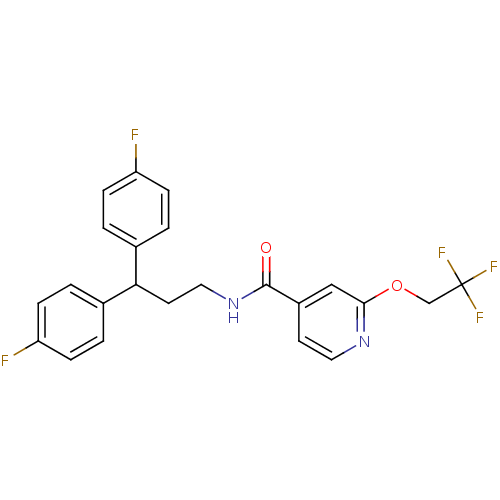 (CHEMBL564893 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297417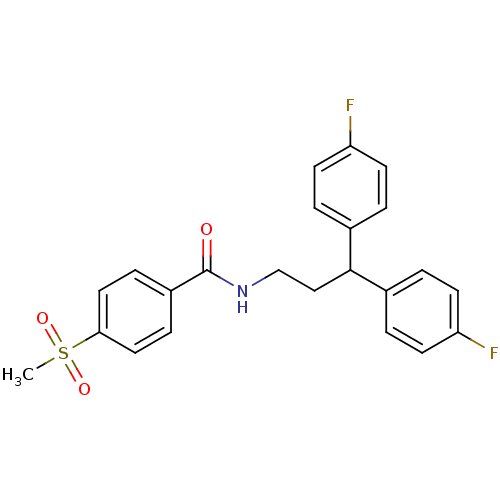 (CHEMBL551338 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297402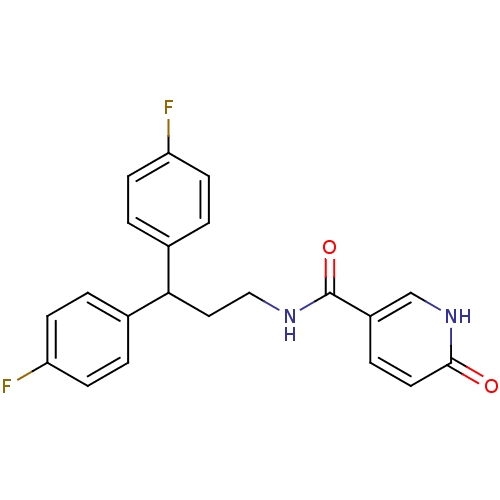 (CHEMBL563417 | N-[4,4-Bis-(4-fluorophenyl)-propyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297412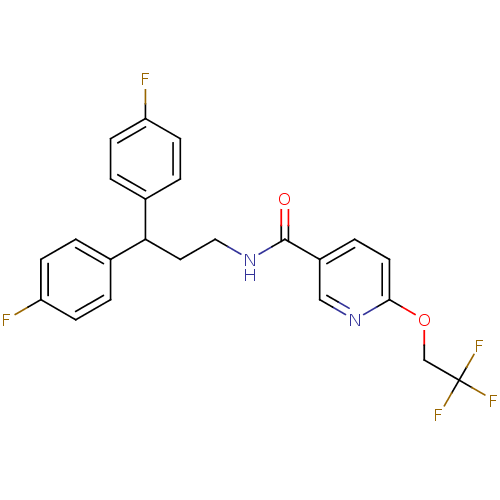 (CHEMBL560740 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297416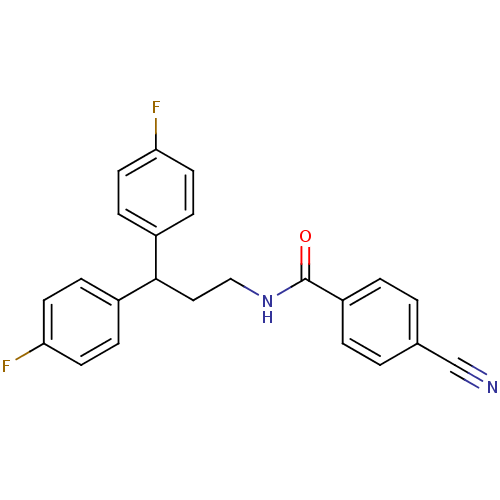 (4-Cyano-N-[3,3-bis-(4-fluorophenyl)-propyl]-benzam...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297396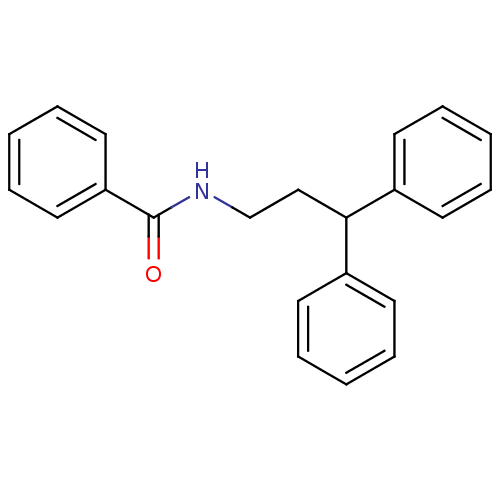 (CHEMBL556303 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50302473 (CHEMBL565628 | N-(3,3-diphenylpropyl)-6-(2,2,2-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble EH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302476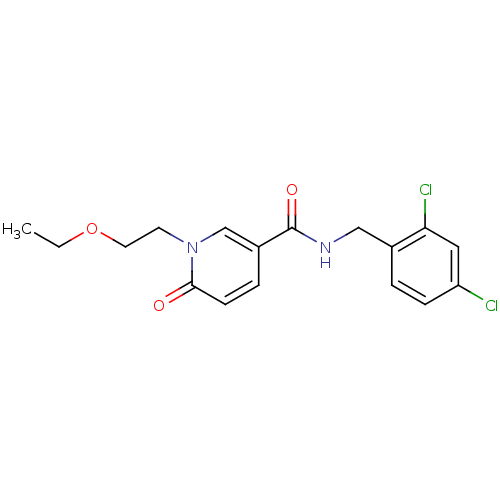 (CHEMBL567283 | N-(2,4-dichlorobenzyl)-1-(2-ethoxye...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297400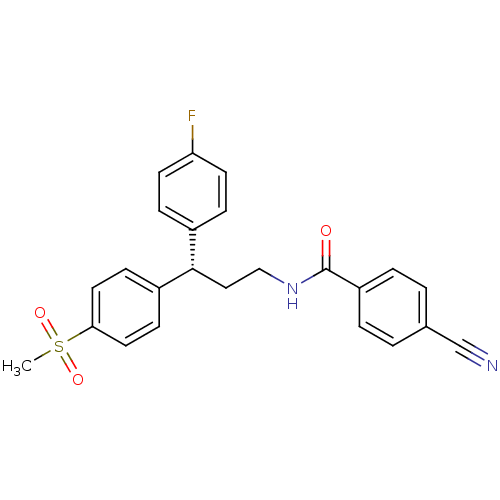 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50414745 (CHEMBL2021549) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50249878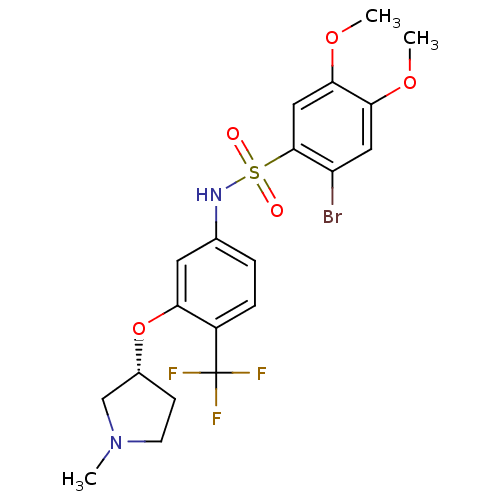 ((R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-U2 from human recombinant urotensin2 receptor expressed in human Chem-2 cells after 4 hrs by scintillation proximity assay | Bioorg Med Chem Lett 23: 2177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.01.105 BindingDB Entry DOI: 10.7270/Q2XS5WSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302464 (CHEMBL567110 | N-(2-(trifluoromethoxy)benzyl)-6-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297412 (CHEMBL560740 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297414 (CHEMBL556234 | N-[3-(4-Fluorophenyl)-3-(4-methanes...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50302474 (CHEMBL572205 | N-(2,4-dichlorobenzyl)-6-(2,2,2-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble EH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302471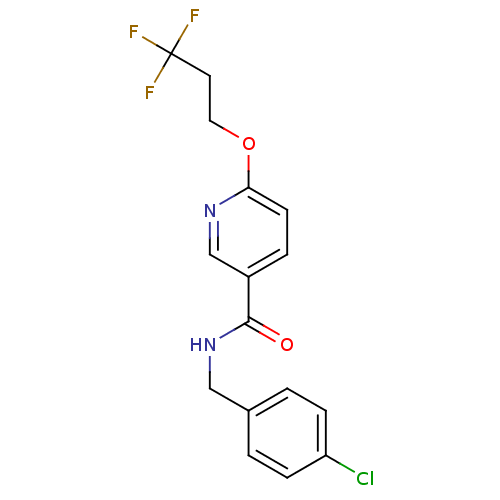 (CHEMBL565841 | N-(4-chlorobenzyl)-6-(3,3,3-trifluo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297417 (CHEMBL551338 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (RAT) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center Curated by ChEMBL | Assay Description Binding to Estrogen receptor- alpha (ER alpha) receptor | Bioorg Med Chem Lett 12: 2875-8 (2002) BindingDB Entry DOI: 10.7270/Q2F18Z2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297398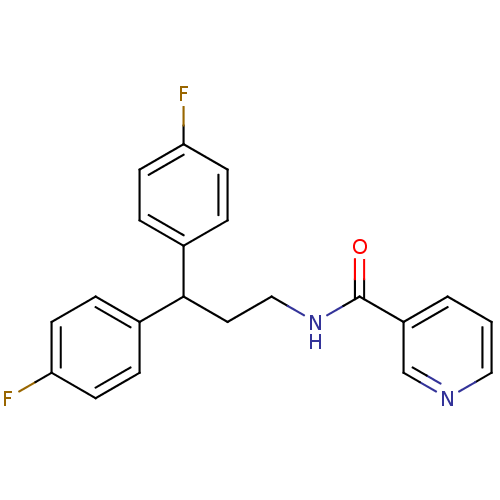 (CHEMBL563216 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297413 (CHEMBL560537 | N-(3,3-diphenyl-propyl)-nicotinamid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble EH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50319983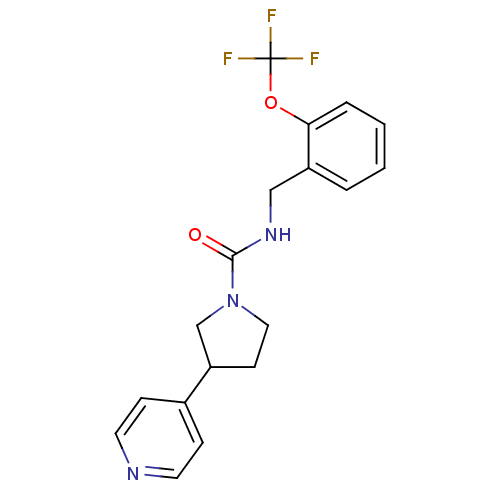 (3-(pyridin-4-yl)-N-(2-(trifluoromethoxy)benzyl)pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase | Bioorg Med Chem Lett 20: 3703-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.078 BindingDB Entry DOI: 10.7270/Q2BV7GTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50414745 (CHEMBL2021549) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297415 (CHEMBL564893 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302462 (CHEMBL566648 | N-(4-bromo-2-(trifluoromethoxy)benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297416 (4-Cyano-N-[3,3-bis-(4-fluorophenyl)-propyl]-benzam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297413 (CHEMBL560537 | N-(3,3-diphenyl-propyl)-nicotinamid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297401 (CHEMBL562081 | N-[3-(4-Fluorophenyl)-3-(4-methanes...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297396 (CHEMBL556303 | N-[3,3-Bis-(4-fluorophenyl)-propyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302474 (CHEMBL572205 | N-(2,4-dichlorobenzyl)-6-(2,2,2-tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297397 (CHEMBL571898 | N-(3,3-Diphenyl-propyl)-isonicotina...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50302473 (CHEMBL565628 | N-(3,3-diphenylpropyl)-6-(2,2,2-tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat sEH | Bioorg Med Chem Lett 19: 5864-8 (2009) Article DOI: 10.1016/j.bmcl.2009.08.074 BindingDB Entry DOI: 10.7270/Q27944SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297397 (CHEMBL571898 | N-(3,3-Diphenyl-propyl)-isonicotina...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297399 (CHEMBL564145 | N-[3-(4-Fluorophenyl)-3-(4-methanes...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 560 total ) | Next | Last >> |