| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Ligand | BDBM50326155 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_658721 (CHEMBL1248462) |
|---|
| IC50 | 82±n/a nM |
|---|
| Citation |  Lakshminarayana, N; Prasad, YR; Gharat, L; Thomas, A; Narayanan, S; Raghuram, A; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel dibenzo[b,d]furan carboxylic acids as potential anti-diabetic agents. Eur J Med Chem45:3709-18 (2010) [PubMed] Article Lakshminarayana, N; Prasad, YR; Gharat, L; Thomas, A; Narayanan, S; Raghuram, A; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel dibenzo[b,d]furan carboxylic acids as potential anti-diabetic agents. Eur J Med Chem45:3709-18 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Name: | Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Synonyms: | PTN1_HUMAN | PTP1B | PTPN1 | Protein tyrosine phosphatase 1B (PTP1B) | Protein tyrosine phosphatase-1B (PTP1B) | Protein-tyrosine phosphatase 1B | Protein-tyrosine phosphatase 1B (PTP1B) | Tyrosine-protein phosphatase non-receptor type 1 | Tyrosine-protein phosphatase non-receptor type 1 (PTP1B) |
|---|
| Type: | Protein phosphatase |
|---|
| Mol. Mass.: | 49963.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human recombinant GST-fusion PTP1B (1-435). |
|---|
| Residue: | 435 |
|---|
| Sequence: | MEMEKEFEQIDKSGSWAAIYQDIRHEASDFPCRVAKLPKNKNRNRYRDVSPFDHSRIKLH
QEDNDYINASLIKMEEAQRSYILTQGPLPNTCGHFWEMVWEQKSRGVVMLNRVMEKGSLK
CAQYWPQKEEKEMIFEDTNLKLTLISEDIKSYYTVRQLELENLTTQETREILHFHYTTWP
DFGVPESPASFLNFLFKVRESGSLSPEHGPVVVHCSAGIGRSGTFCLADTCLLLMDKRKD
PSSVDIKKVLLEMRKFRMGLIQTADQLRFSYLAVIEGAKFIMGDSSVQDQWKELSHEDLE
PPPEHIPPPPRPPKRILEPHNGKCREFFPNHQWVKEETQEDKDCPIKEEKGSPLNAAPYG
IESMSQDTEVRSRVVGGSLRGAQAASPAKGEPSLPEKDEDHALSYWKPFLVNMCVATVLT
AGAYLCYRFLFNSNT
|
|
|
|---|
| BDBM50326155 |
|---|
| n/a |
|---|
| Name | BDBM50326155 |
|---|
| Synonyms: | 8-{2-[4-(Pyrrolidin-1-yl)phenyl]thiazol-4-yl}dibenzo[b,d]furan-4-carboxylic acid | CHEMBL1242362 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H20N2O3S |
|---|
| Mol. Mass. | 440.514 |
|---|
| SMILES | OC(=O)c1cccc2c3cc(ccc3oc12)-c1csc(n1)-c1ccc(cc1)N1CCCC1 |
|---|
| Structure | 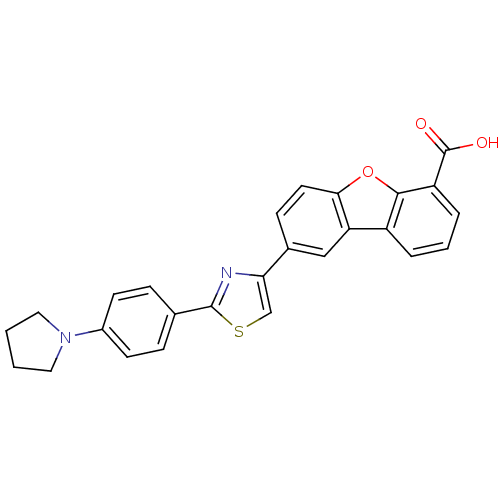
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lakshminarayana, N; Prasad, YR; Gharat, L; Thomas, A; Narayanan, S; Raghuram, A; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel dibenzo[b,d]furan carboxylic acids as potential anti-diabetic agents. Eur J Med Chem45:3709-18 (2010) [PubMed] Article
Lakshminarayana, N; Prasad, YR; Gharat, L; Thomas, A; Narayanan, S; Raghuram, A; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel dibenzo[b,d]furan carboxylic acids as potential anti-diabetic agents. Eur J Med Chem45:3709-18 (2010) [PubMed] Article