 Found 153 hits with Last Name = 'gopalan' and Initial = 'b'
Found 153 hits with Last Name = 'gopalan' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326165
(CHEMBL1241315 | oxalylaminobenzoic acid)Show SMILES CCc1cc(C[C@H]2NC(=O)CN(CCCCOc3cccc(O)c3C(=O)OC)C2=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H35N3O11/c1-3-21-17-20(13-14-24(21)37(31(41)33(44)45)25-10-5-4-9-22(25)32(42)43)18-23-30(40)36(19-28(39)35-23)15-6-7-16-48-27-12-8-11-26(38)29(27)34(46)47-2/h4-5,8-14,17,23,38H,3,6-7,15-16,18-19H2,1-2H3,(H,35,39)(H,42,43)(H,44,45)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50326165

(CHEMBL1241315 | oxalylaminobenzoic acid)Show SMILES CCc1cc(C[C@H]2NC(=O)CN(CCCCOc3cccc(O)c3C(=O)OC)C2=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H35N3O11/c1-3-21-17-20(13-14-24(21)37(31(41)33(44)45)25-10-5-4-9-22(25)32(42)43)18-23-30(40)36(19-28(39)35-23)15-6-7-16-48-27-12-8-11-26(38)29(27)34(46)47-2/h4-5,8-14,17,23,38H,3,6-7,15-16,18-19H2,1-2H3,(H,35,39)(H,42,43)(H,44,45)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50296368
(7-(biphenyl-4-yl)-4-oxo-4H-chromene-3-carbaldehyde...)Show InChI InChI=1S/C22H14O3/c23-13-19-14-25-21-12-18(10-11-20(21)22(19)24)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296368

(7-(biphenyl-4-yl)-4-oxo-4H-chromene-3-carbaldehyde...)Show InChI InChI=1S/C22H14O3/c23-13-19-14-25-21-12-18(10-11-20(21)22(19)24)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50133280
(5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H17NO7/c1-27-21(26)19-16(23)8-3-9-17(19)28-10-4-6-13-5-2-7-14(11-13)18-12-15(20(24)25)22-29-18/h2-9,11-12,23H,10H2,1H3,(H,24,25)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50133280

(5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H17NO7/c1-27-21(26)19-16(23)8-3-9-17(19)28-10-4-6-13-5-2-7-14(11-13)18-12-15(20(24)25)22-29-18/h2-9,11-12,23H,10H2,1H3,(H,24,25)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50296368

(7-(biphenyl-4-yl)-4-oxo-4H-chromene-3-carbaldehyde...)Show InChI InChI=1S/C22H14O3/c23-13-19-14-25-21-12-18(10-11-20(21)22(19)24)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296368

(7-(biphenyl-4-yl)-4-oxo-4H-chromene-3-carbaldehyde...)Show InChI InChI=1S/C22H14O3/c23-13-19-14-25-21-12-18(10-11-20(21)22(19)24)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50133280

(5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H17NO7/c1-27-21(26)19-16(23)8-3-9-17(19)28-10-4-6-13-5-2-7-14(11-13)18-12-15(20(24)25)22-29-18/h2-9,11-12,23H,10H2,1H3,(H,24,25)/b6-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50133280

(5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H17NO7/c1-27-21(26)19-16(23)8-3-9-17(19)28-10-4-6-13-5-2-7-14(11-13)18-12-15(20(24)25)22-29-18/h2-9,11-12,23H,10H2,1H3,(H,24,25)/b6-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326165

(CHEMBL1241315 | oxalylaminobenzoic acid)Show SMILES CCc1cc(C[C@H]2NC(=O)CN(CCCCOc3cccc(O)c3C(=O)OC)C2=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H35N3O11/c1-3-21-17-20(13-14-24(21)37(31(41)33(44)45)25-10-5-4-9-22(25)32(42)43)18-23-30(40)36(19-28(39)35-23)15-6-7-16-48-27-12-8-11-26(38)29(27)34(46)47-2/h4-5,8-14,17,23,38H,3,6-7,15-16,18-19H2,1-2H3,(H,35,39)(H,42,43)(H,44,45)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296382
((3S)-7-{4-[(3R)-1,2-Dithiolan-3-yl]butylcarboxamid...)Show SMILES OC(=O)[C@@H]1Cc2ccc(NC(=O)CCCC[C@@H]3CCSS3)cc2CO1 |r| Show InChI InChI=1S/C18H23NO4S2/c20-17(4-2-1-3-15-7-8-24-25-15)19-14-6-5-12-10-16(18(21)22)23-11-13(12)9-14/h5-6,9,15-16H,1-4,7-8,10-11H2,(H,19,20)(H,21,22)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296382

((3S)-7-{4-[(3R)-1,2-Dithiolan-3-yl]butylcarboxamid...)Show SMILES OC(=O)[C@@H]1Cc2ccc(NC(=O)CCCC[C@@H]3CCSS3)cc2CO1 |r| Show InChI InChI=1S/C18H23NO4S2/c20-17(4-2-1-3-15-7-8-24-25-15)19-14-6-5-12-10-16(18(21)22)23-11-13(12)9-14/h5-6,9,15-16H,1-4,7-8,10-11H2,(H,19,20)(H,21,22)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296381
((3S)-6-{4-[(3R)-1,2-Dithiolan-3-yl]butylcarboxamid...)Show SMILES OC(=O)[C@@H]1Cc2cc(NC(=O)CCCC[C@@H]3CCSS3)ccc2CO1 |r| Show InChI InChI=1S/C18H23NO4S2/c20-17(4-2-1-3-15-7-8-24-25-15)19-14-6-5-12-11-23-16(18(21)22)10-13(12)9-14/h5-6,9,15-16H,1-4,7-8,10-11H2,(H,19,20)(H,21,22)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326155
(8-{2-[4-(Pyrrolidin-1-yl)phenyl]thiazol-4-yl}diben...)Show SMILES OC(=O)c1cccc2c3cc(ccc3oc12)-c1csc(n1)-c1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C26H20N2O3S/c29-26(30)20-5-3-4-19-21-14-17(8-11-23(21)31-24(19)20)22-15-32-25(27-22)16-6-9-18(10-7-16)28-12-1-2-13-28/h3-11,14-15H,1-2,12-13H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296380
((3S)-7-[4-(1,2-Dithiolan-3-yl)butylcarboxamido]-is...)Show SMILES OC(=O)[C@@H]1Cc2ccc(NC(=O)CCCCC3CCSS3)cc2CO1 |r| Show InChI InChI=1S/C18H23NO4S2/c20-17(4-2-1-3-15-7-8-24-25-15)19-14-6-5-12-10-16(18(21)22)23-11-13(12)9-14/h5-6,9,15-16H,1-4,7-8,10-11H2,(H,19,20)(H,21,22)/t15?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296379

((3S)-6-[4-(1,2-Dithiolan-3-yl)butylcarboxamido]-is...)Show SMILES OC(=O)[C@@H]1Cc2cc(NC(=O)CCCCC3CCSS3)ccc2CO1 |r| Show InChI InChI=1S/C18H23NO4S2/c20-17(4-2-1-3-15-7-8-24-25-15)19-14-6-5-12-11-23-16(18(21)22)10-13(12)9-14/h5-6,9,15-16H,1-4,7-8,10-11H2,(H,19,20)(H,21,22)/t15?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296391
((2S)-3-(4-{4-[(3R)-1,2-Dithiolan-3-yl]butylcarboxa...)Show SMILES CCO[C@@H](Cc1ccc(NC(=O)CCCC[C@@H]2CCSS2)cc1)C(O)=O |r| Show InChI InChI=1S/C19H27NO4S2/c1-2-24-17(19(22)23)13-14-7-9-15(10-8-14)20-18(21)6-4-3-5-16-11-12-25-26-16/h7-10,16-17H,2-6,11-13H2,1H3,(H,20,21)(H,22,23)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296386

((3S)-7-{4-[(4R)-1,3-Dithian-4-yl]butylcarboxamido}...)Show SMILES OC(=O)[C@@H]1Cc2ccc(NC(=O)CCCC[C@@H]3CCSCS3)cc2CO1 |r| Show InChI InChI=1S/C19H25NO4S2/c21-18(4-2-1-3-16-7-8-25-12-26-16)20-15-6-5-13-10-17(19(22)23)24-11-14(13)9-15/h5-6,9,16-17H,1-4,7-8,10-12H2,(H,20,21)(H,22,23)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326156

(8-{2-[4-(Piperidin-1-yl)phenyl]thiazol-4-yl}dibenz...)Show SMILES OC(=O)c1cccc2c3cc(ccc3oc12)-c1csc(n1)-c1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C27H22N2O3S/c30-27(31)21-6-4-5-20-22-15-18(9-12-24(22)32-25(20)21)23-16-33-26(28-23)17-7-10-19(11-8-17)29-13-2-1-3-14-29/h4-12,15-16H,1-3,13-14H2,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296376

((3S)-7-[4-(2,2-Dimethyl-1,3-dithiolan-4-yl)butylca...)Show SMILES CC1(C)SCC(CCCCC(=O)Nc2ccc3C[C@H](OCc3c2)C(O)=O)S1 |r| Show InChI InChI=1S/C20H27NO4S2/c1-20(2)26-12-16(27-20)5-3-4-6-18(22)21-15-8-7-13-10-17(19(23)24)25-11-14(13)9-15/h7-9,16-17H,3-6,10-12H2,1-2H3,(H,21,22)(H,23,24)/t16?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50326165

(CHEMBL1241315 | oxalylaminobenzoic acid)Show SMILES CCc1cc(C[C@H]2NC(=O)CN(CCCCOc3cccc(O)c3C(=O)OC)C2=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H35N3O11/c1-3-21-17-20(13-14-24(21)37(31(41)33(44)45)25-10-5-4-9-22(25)32(42)43)18-23-30(40)36(19-28(39)35-23)15-6-7-16-48-27-12-8-11-26(38)29(27)34(46)47-2/h4-5,8-14,17,23,38H,3,6-7,15-16,18-19H2,1-2H3,(H,35,39)(H,42,43)(H,44,45)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296390
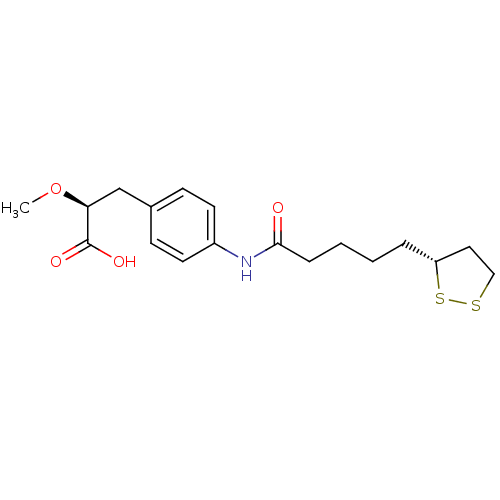
((2S)-3-(4-{4-[(3R)-1,2-Dithiolan-3-yl]butylcarboxa...)Show SMILES CO[C@@H](Cc1ccc(NC(=O)CCCC[C@@H]2CCSS2)cc1)C(O)=O |r| Show InChI InChI=1S/C18H25NO4S2/c1-23-16(18(21)22)12-13-6-8-14(9-7-13)19-17(20)5-3-2-4-15-10-11-24-25-15/h6-9,15-16H,2-5,10-12H2,1H3,(H,19,20)(H,21,22)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296375

((3S)-6-[4-(2,2-Dimethyl-1,3-dithiolan-4-yl)butylca...)Show SMILES CC1(C)SCC(CCCCC(=O)Nc2ccc3CO[C@@H](Cc3c2)C(O)=O)S1 |r| Show InChI InChI=1S/C20H27NO4S2/c1-20(2)26-12-16(27-20)5-3-4-6-18(22)21-15-8-7-13-11-25-17(19(23)24)10-14(13)9-15/h7-9,16-17H,3-6,10-12H2,1-2H3,(H,21,22)(H,23,24)/t16?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296385
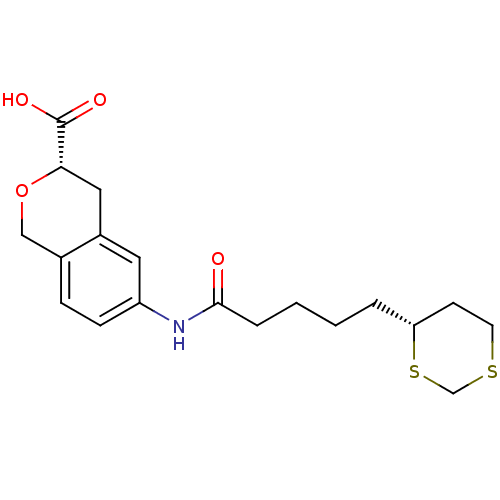
((3S)-6-{4-[(4R)-1,3-Dithian-4-yl]butylcarboxamido}...)Show SMILES OC(=O)[C@@H]1Cc2cc(NC(=O)CCCC[C@@H]3CCSCS3)ccc2CO1 |r| Show InChI InChI=1S/C19H25NO4S2/c21-18(4-2-1-3-16-7-8-25-12-26-16)20-15-6-5-13-11-24-17(19(22)23)10-14(13)9-15/h5-6,9,16-17H,1-4,7-8,10-12H2,(H,20,21)(H,22,23)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326160
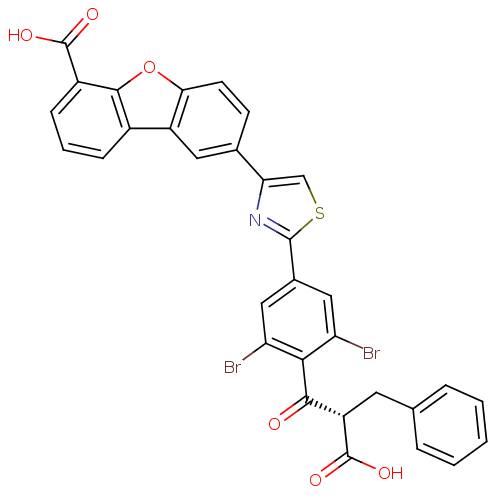
(8-[2-(3,5-Dibromo-4-(1-carboxy-2-phenylethoxy)phen...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)C(=O)c1c(Br)cc(cc1Br)-c1nc(cs1)-c1ccc2oc3c(cccc3c2c1)C(O)=O |r| Show InChI InChI=1S/C32H19Br2NO6S/c33-23-13-18(14-24(34)27(23)28(36)22(32(39)40)11-16-5-2-1-3-6-16)30-35-25(15-42-30)17-9-10-26-21(12-17)19-7-4-8-20(31(37)38)29(19)41-26/h1-10,12-15,22H,11H2,(H,37,38)(H,39,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326153

(8-[2-(4-Aminophenyl)thiazol-4-yl]dibenzo[b,d]furan...)Show SMILES Nc1ccc(cc1)-c1nc(cs1)-c1ccc2oc3c(cccc3c2c1)C(O)=O Show InChI InChI=1S/C22H14N2O3S/c23-14-7-4-12(5-8-14)21-24-18(11-28-21)13-6-9-19-17(10-13)15-2-1-3-16(22(25)26)20(15)27-19/h1-11H,23H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326158

(8-{2-[4-(2,6-Dimethylmorpholino)phenyl]thiazol-4-y...)Show SMILES CC1CN(CC(C)O1)c1ccc(cc1)-c1nc(cs1)-c1ccc2oc3c(cccc3c2c1)C(O)=O Show InChI InChI=1S/C28H24N2O4S/c1-16-13-30(14-17(2)33-16)20-9-6-18(7-10-20)27-29-24(15-35-27)19-8-11-25-23(12-19)21-4-3-5-22(28(31)32)26(21)34-25/h3-12,15-17H,13-14H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326152
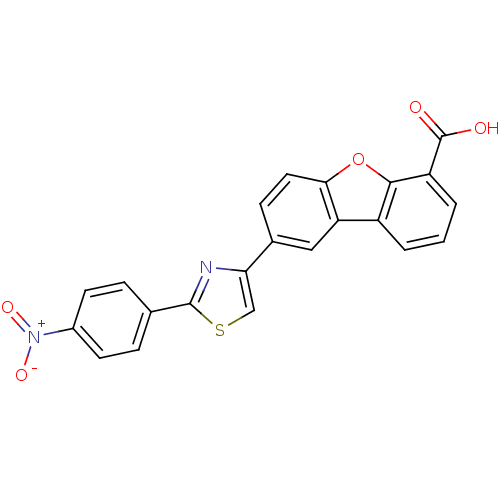
(8-[2-(4-Nitrophenyl)thiazol-4-yl]dibenzo[b,d]furan...)Show SMILES OC(=O)c1cccc2c3cc(ccc3oc12)-c1csc(n1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C22H12N2O5S/c25-22(26)16-3-1-2-15-17-10-13(6-9-19(17)29-20(15)16)18-11-30-21(23-18)12-4-7-14(8-5-12)24(27)28/h1-11H,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326154
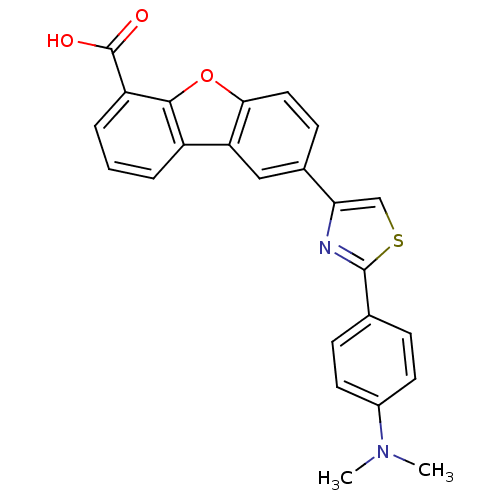
(8-{2-[4-(Dimethylamino)phenyl]thiazol-4-yl}dibenzo...)Show SMILES CN(C)c1ccc(cc1)-c1nc(cs1)-c1ccc2oc3c(cccc3c2c1)C(O)=O Show InChI InChI=1S/C24H18N2O3S/c1-26(2)16-9-6-14(7-10-16)23-25-20(13-30-23)15-8-11-21-19(12-15)17-4-3-5-18(24(27)28)22(17)29-21/h3-13H,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326157
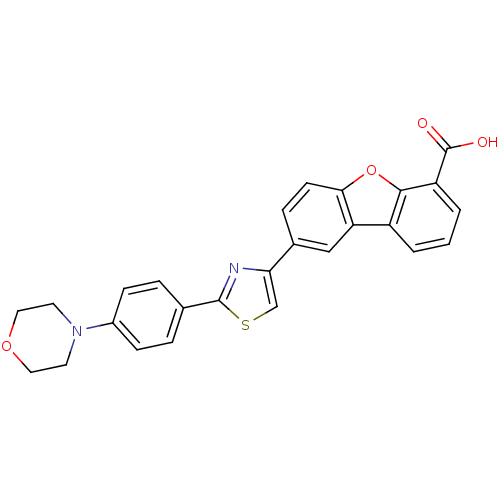
(8-[2-(4-Morpholinophenyl)thiazol-4-yl]dibenzo[b,d]...)Show SMILES OC(=O)c1cccc2c3cc(ccc3oc12)-c1csc(n1)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C26H20N2O4S/c29-26(30)20-3-1-2-19-21-14-17(6-9-23(21)32-24(19)20)22-15-33-25(27-22)16-4-7-18(8-5-16)28-10-12-31-13-11-28/h1-9,14-15H,10-13H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296389
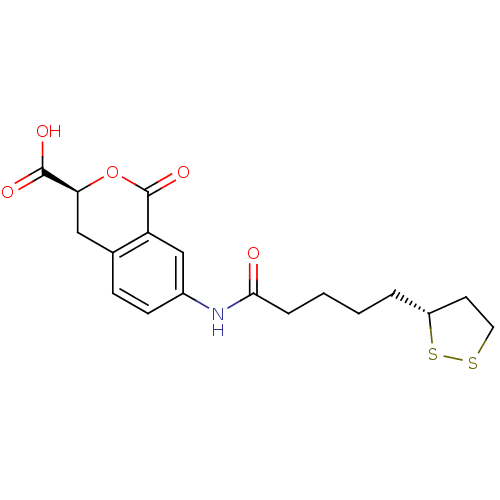
((3S)-7-{4-[(3R)-1,2-Dithiolan-3-yl]butylcarboxamid...)Show SMILES OC(=O)[C@@H]1Cc2ccc(NC(=O)CCCC[C@@H]3CCSS3)cc2C(=O)O1 |r| Show InChI InChI=1S/C18H21NO5S2/c20-16(4-2-1-3-13-7-8-25-26-13)19-12-6-5-11-9-15(17(21)22)24-18(23)14(11)10-12/h5-6,10,13,15H,1-4,7-9H2,(H,19,20)(H,21,22)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50143215

(CHEMBL3758457)Show SMILES ONC(=O)\C=C\c1ccc(\C=C(\CNC2CC2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H21FN2O2/c22-19-8-6-17(7-9-19)18(14-23-20-10-11-20)13-16-3-1-15(2-4-16)5-12-21(25)24-26/h1-9,12-13,20,23,26H,10-11,14H2,(H,24,25)/b12-5+,18-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Orchid Chemicals& Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) |
Eur J Med Chem 108: 274-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.014
BindingDB Entry DOI: 10.7270/Q2J9687D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50296376

((3S)-7-[4-(2,2-Dimethyl-1,3-dithiolan-4-yl)butylca...)Show SMILES CC1(C)SCC(CCCCC(=O)Nc2ccc3C[C@H](OCc3c2)C(O)=O)S1 |r| Show InChI InChI=1S/C20H27NO4S2/c1-20(2)26-12-16(27-20)5-3-4-6-18(22)21-15-8-7-13-10-17(19(23)24)25-11-14(13)9-15/h7-9,16-17H,3-6,10-12H2,1-2H3,(H,21,22)(H,23,24)/t16?,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50296375

((3S)-6-[4-(2,2-Dimethyl-1,3-dithiolan-4-yl)butylca...)Show SMILES CC1(C)SCC(CCCCC(=O)Nc2ccc3CO[C@@H](Cc3c2)C(O)=O)S1 |r| Show InChI InChI=1S/C20H27NO4S2/c1-20(2)26-12-16(27-20)5-3-4-6-18(22)21-15-8-7-13-11-25-17(19(23)24)10-14(13)9-15/h7-9,16-17H,3-6,10-12H2,1-2H3,(H,21,22)(H,23,24)/t16?,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50326152

(8-[2-(4-Nitrophenyl)thiazol-4-yl]dibenzo[b,d]furan...)Show SMILES OC(=O)c1cccc2c3cc(ccc3oc12)-c1csc(n1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C22H12N2O5S/c25-22(26)16-3-1-2-15-17-10-13(6-9-19(17)29-20(15)16)18-11-30-21(23-18)12-4-7-14(8-5-12)24(27)28/h1-11H,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50296374
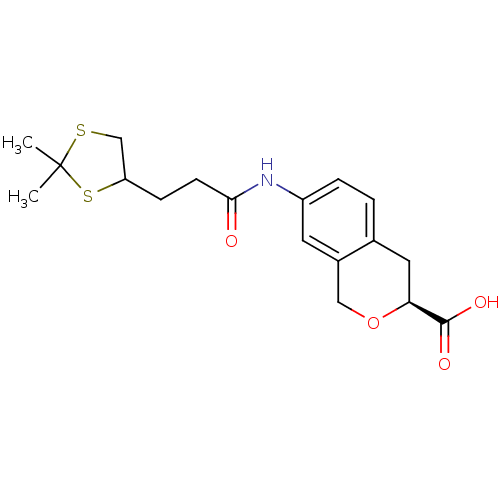
((3S)-7-[2-(2,2-Dimethyl-1,3-dithiolan-4-yl)ethylca...)Show SMILES CC1(C)SCC(CCC(=O)Nc2ccc3C[C@H](OCc3c2)C(O)=O)S1 |r| Show InChI InChI=1S/C18H23NO4S2/c1-18(2)24-10-14(25-18)5-6-16(20)19-13-4-3-11-8-15(17(21)22)23-9-12(11)7-13/h3-4,7,14-15H,5-6,8-10H2,1-2H3,(H,19,20)(H,21,22)/t14?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 354 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data