

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50326152 8-[2-(4-Nitrophenyl)thiazol-4-yl]dibenzo[b,d]furan-4-carboxylic acid::CHEMBL1242552
SMILES: OC(=O)c1cccc2c3cc(ccc3oc12)-c1csc(n1)-c1ccc(cc1)[N+]([O-])=O
InChI Key: InChIKey=FHNSBIYBHXTXGH-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50326152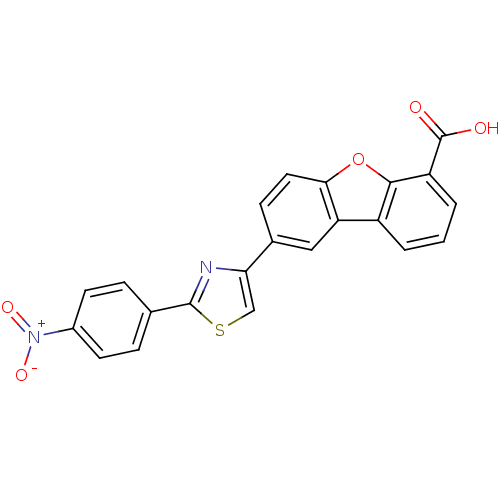 (8-[2-(4-Nitrophenyl)thiazol-4-yl]dibenzo[b,d]furan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry | Eur J Med Chem 45: 3709-18 (2010) Article DOI: 10.1016/j.ejmech.2010.05.020 BindingDB Entry DOI: 10.7270/Q24Q7V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (LAR) (Homo sapiens (Human)) | BDBM50326152 (8-[2-(4-Nitrophenyl)thiazol-4-yl]dibenzo[b,d]furan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant LAR after 30 mins by spectrophotometry | Eur J Med Chem 45: 3709-18 (2010) Article DOI: 10.1016/j.ejmech.2010.05.020 BindingDB Entry DOI: 10.7270/Q24Q7V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50326152 (8-[2-(4-Nitrophenyl)thiazol-4-yl]dibenzo[b,d]furan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry | Eur J Med Chem 45: 3709-18 (2010) Article DOI: 10.1016/j.ejmech.2010.05.020 BindingDB Entry DOI: 10.7270/Q24Q7V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||