

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Insulin-like growth factor 1 receptor | ||
| Ligand | BDBM50327947 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_665190 (CHEMBL1260609) | ||
| IC50 | 87±n/a nM | ||
| Citation |  Wang, GT; Mantei, RA; Hubbard, RD; Wilsbacher, JL; Zhang, Q; Tucker, L; Hu, X; Kovar, P; Johnson, EF; Osterling, DJ; Bouska, J; Wang, J; Davidsen, SK; Bell, RL; Sheppard, GS Substituted 4-amino-1H-pyrazolo[3,4-d]pyrimidines as multi-targeted inhibitors of insulin-like growth factor-1 receptor (IGF1R) and members of ErbB-family receptor kinases. Bioorg Med Chem Lett20:6067-71 (2010) [PubMed] Article Wang, GT; Mantei, RA; Hubbard, RD; Wilsbacher, JL; Zhang, Q; Tucker, L; Hu, X; Kovar, P; Johnson, EF; Osterling, DJ; Bouska, J; Wang, J; Davidsen, SK; Bell, RL; Sheppard, GS Substituted 4-amino-1H-pyrazolo[3,4-d]pyrimidines as multi-targeted inhibitors of insulin-like growth factor-1 receptor (IGF1R) and members of ErbB-family receptor kinases. Bioorg Med Chem Lett20:6067-71 (2010) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Insulin-like growth factor 1 receptor | |||
| Name: | Insulin-like growth factor 1 receptor | ||
| Synonyms: | CD_antigen=CD221 | IGF-I receptor | IGF1R | IGF1R_HUMAN | Insulin-like growth factor 1 receptor (IGF1R) | Insulin-like growth factor 1 receptor (IGFIR) | Insulin-like growth factor 1 receptor alpha chain | Insulin-like growth factor 1 receptor beta chain | Insulin-like growth factor I receptor | Insulin-like growth factor receptor (IGFR) | ||
| Type: | Protein | ||
| Mol. Mass.: | 154776.79 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P08069 | ||
| Residue: | 1367 | ||
| Sequence: |
| ||
| BDBM50327947 | |||
| n/a | |||
| Name | BDBM50327947 | ||
| Synonyms: | CHEMBL1256432 | trans-N-(4-(4-amino-1-(4-(4-methylpiperazin-1-yl)cyclohexyl)-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-2-fluorophenyl)-7-chlorobenzo[d]oxazol-2-amine | ||
| Type | Small organic molecule | ||
| Emp. Form. | C29H31ClFN9O | ||
| Mol. Mass. | 576.068 | ||
| SMILES | CN1CCN(CC1)[C@H]1CC[C@@H](CC1)n1nc(-c2ccc(Nc3nc4cccc(Cl)c4o3)c(F)c2)c2c(N)ncnc12 |r,wU:10.14,wD:7.7,(-3.03,-35.57,;-3.49,-34.1,;-5,-33.77,;-5.46,-32.31,;-4.43,-31.17,;-2.92,-31.49,;-2.45,-32.96,;-4.9,-29.71,;-3.86,-28.56,;-4.33,-27.09,;-5.84,-26.77,;-6.87,-27.92,;-6.4,-29.38,;-6.32,-25.31,;-5.42,-24.07,;-6.32,-22.82,;-5.55,-21.49,;-4.01,-21.49,;-3.24,-20.16,;-4.02,-18.82,;-3.25,-17.49,;-1.71,-17.48,;-.81,-16.22,;.66,-16.69,;1.97,-15.92,;3.3,-16.68,;3.31,-18.22,;1.98,-18.99,;1.99,-20.53,;.66,-18.23,;-.8,-18.71,;-5.56,-18.83,;-6.34,-17.5,;-6.32,-20.17,;-7.78,-23.3,;-9.11,-22.53,;-9.11,-20.99,;-10.45,-23.3,;-10.45,-24.84,;-9.11,-25.61,;-7.78,-24.84,)| | ||
| Structure | 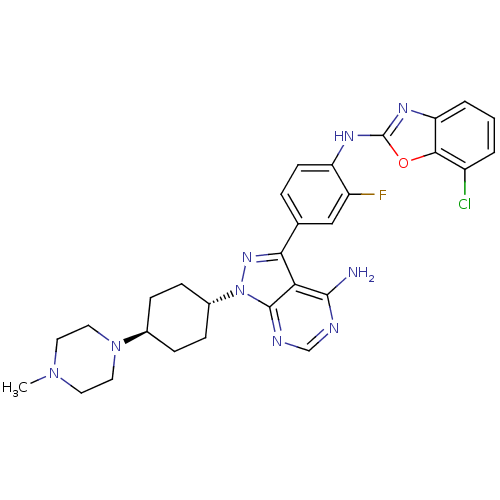
| ||