| Reaction Details |
|---|
 | Report a problem with these data |
| Target | DNA gyrase subunit B |
|---|
| Ligand | BDBM50330295 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_677263 (CHEMBL1279101) |
|---|
| IC50 | 758±n/a nM |
|---|
| Citation |  Anderle, C; Stieger, M; Burrell, M; Reinelt, S; Maxwell, A; Page, M; Heide, L Biological activities of novel gyrase inhibitors of the aminocoumarin class. Antimicrob Agents Chemother52:1982-90 (2008) [PubMed] Article Anderle, C; Stieger, M; Burrell, M; Reinelt, S; Maxwell, A; Page, M; Heide, L Biological activities of novel gyrase inhibitors of the aminocoumarin class. Antimicrob Agents Chemother52:1982-90 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| DNA gyrase subunit B |
|---|
| Name: | DNA gyrase subunit B |
|---|
| Synonyms: | DNA gyrase subunit B | DNA gyrase subunit B (gyrB) | GYRB_ECOLI | Type IIA topoisomerase subunit GyrB | acrB | cou | gyrB | himB | hisU | nalC | parA | pcbA |
|---|
| Type: | Enzyme Subunit |
|---|
| Mol. Mass.: | 89941.28 |
|---|
| Organism: | Escherichia coli (strain K12) |
|---|
| Description: | P0AES6 |
|---|
| Residue: | 804 |
|---|
| Sequence: | MSNSYDSSSIKVLKGLDAVRKRPGMYIGDTDDGTGLHHMVFEVVDNAIDEALAGHCKEII
VTIHADNSVSVQDDGRGIPTGIHPEEGVSAAEVIMTVLHAGGKFDDNSYKVSGGLHGVGV
SVVNALSQKLELVIQREGKIHRQIYEHGVPQAPLAVTGETEKTGTMVRFWPSLETFTNVT
EFEYEILAKRLRELSFLNSGVSIRLRDKRDGKEDHFHYEGGIKAFVEYLNKNKTPIHPNI
FYFSTEKDGIGVEVALQWNDGFQENIYCFTNNIPQRDGGTHLAGFRAAMTRTLNAYMDKE
GYSKKAKVSATGDDAREGLIAVVSVKVPDPKFSSQTKDKLVSSEVKSAVEQQMNELLAEY
LLENPTDAKIVVGKIIDAARAREAARRAREMTRRKGALDLAGLPGKLADCQERDPALSEL
YLVEGDSAGGSAKQGRNRKNQAILPLKGKILNVEKARFDKMLSSQEVATLITALGCGIGR
DEYNPDKLRYHSIIIMTDADVDGSHIRTLLLTFFYRQMPEIVERGHVYIAQPPLYKVKKG
KQEQYIKDDEAMDQYQISIALDGATLHTNASAPALAGEALEKLVSEYNATQKMINRMERR
YPKAMLKELIYQPTLTEADLSDEQTVTRWVNALVSELNDKEQHGSQWKFDVHTNAEQNLF
EPIVRVRTHGVDTDYPLDHEFITGGEYRRICTLGEKLRGLLEEDAFIERGERRQPVASFE
QALDWLVKESRRGLSIQRYKGLGEMNPEQLWETTMDPESRRMLRVTVKDAIAADQLFTTL
MGDAVEPRRAFIEENALKAANIDI
|
|
|
|---|
| BDBM50330295 |
|---|
| n/a |
|---|
| Name | BDBM50330295 |
|---|
| Synonyms: | (2R,3R,4R,5R)-4-hydroxy-2-(4-hydroxy-3-(4-hydroxy-3-propylbenzamido)-8-methyl-2-oxo-2H-chromen-7-yloxy)-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-3-yl 5-methyl-1H-pyrrole-2-carboxylate | CHEMBL1275897 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C34H38N2O11 |
|---|
| Mol. Mass. | 650.6723 |
|---|
| SMILES | CCCc1cc(ccc1O)C(=O)Nc1c(O)c2ccc(O[C@@H]3OC(C)(C)[C@H](OC)[C@@H](O)[C@H]3OC(=O)c3ccc(C)[nH]3)c(C)c2oc1=O |r| |
|---|
| Structure | 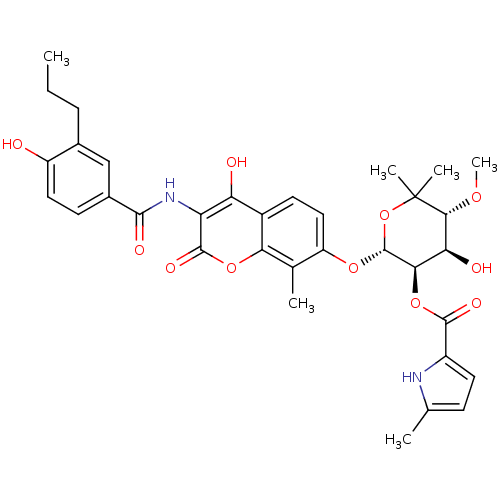
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Anderle, C; Stieger, M; Burrell, M; Reinelt, S; Maxwell, A; Page, M; Heide, L Biological activities of novel gyrase inhibitors of the aminocoumarin class. Antimicrob Agents Chemother52:1982-90 (2008) [PubMed] Article
Anderle, C; Stieger, M; Burrell, M; Reinelt, S; Maxwell, A; Page, M; Heide, L Biological activities of novel gyrase inhibitors of the aminocoumarin class. Antimicrob Agents Chemother52:1982-90 (2008) [PubMed] Article