| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2B6 |
|---|
| Ligand | BDBM50184924 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_686354 (CHEMBL1293110) |
|---|
| IC50 | >10000000±n/a nM |
|---|
| Citation |  Hadden, M; Deering, DM; Henderson, AJ; Surman, MD; Luche, M; Khmelnitsky, Y; Vickers, S; Viggers, J; Cheetham, S; Guzzo, PR Synthesis and SAR of 4-aryl-1-(indazol-5-yl)pyridin-2(1H)ones as MCH-1 antagonists for the treatment of obesity. Bioorg Med Chem Lett20:7020-3 (2010) [PubMed] Article Hadden, M; Deering, DM; Henderson, AJ; Surman, MD; Luche, M; Khmelnitsky, Y; Vickers, S; Viggers, J; Cheetham, S; Guzzo, PR Synthesis and SAR of 4-aryl-1-(indazol-5-yl)pyridin-2(1H)ones as MCH-1 antagonists for the treatment of obesity. Bioorg Med Chem Lett20:7020-3 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2B6 |
|---|
| Name: | Cytochrome P450 2B6 |
|---|
| Synonyms: | CP2B6_HUMAN | CYP2B6 | Cytochrome P450 2B6 (CYP2B6) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56289.75 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P20813 |
|---|
| Residue: | 491 |
|---|
| Sequence: | MELSVLLFLALLTGLLLLLVQRHPNTHDRLPPGPRPLPLLGNLLQMDRRGLLKSFLRFRE
KYGDVFTVHLGPRPVVMLCGVEAIREALVDKAEAFSGRGKIAMVDPFFRGYGVIFANGNR
WKVLRRFSVTTMRDFGMGKRSVEERIQEEAQCLIEELRKSKGALMDPTFLFQSITANIIC
SIVFGKRFHYQDQEFLKMLNLFYQTFSLISSVFGQLFELFSGFLKYFPGAHRQVYKNLQE
INAYIGHSVEKHRETLDPSAPKDLIDTYLLHMEKEKSNAHSEFSHQNLNLNTLSLFFAGT
ETTSTTLRYGFLLMLKYPHVAERVYREIEQVIGPHRPPELHDRAKMPYTEAVIYEIQRFS
DLLPMGVPHIVTQHTSFRGYIIPKDTEVFLILSTALHDPHYFEKPDAFNPDHFLDANGAL
KKTEAFIPFSLGKRICLGEGIARAELFLFFTTILQNFSMASPVAPEDIDLTPQECGVGKI
PPTYQIRFLPR
|
|
|
|---|
| BDBM50184924 |
|---|
| n/a |
|---|
| Name | BDBM50184924 |
|---|
| Synonyms: | 1-((1S,2R,5S)-5-(4-cyanophenyl)bicyclo[3.1.0]hexan-2-yl)-3-(4-fluoro-3-(trifluoromethyl)phenyl)-1-(3-(4-methylpiperazin-1-yl)propyl)urea | CHEMBL205285 | N-[trans-5-(4-cyanophenyl)bicyclo[3.1.0]hex-2-yl]-N'-[4-fluoro-3-(trifluoromethyl)phenyl)-N-[3-(4-methyl-1-piperazinyl)propyl]-urea |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H33F4N5O |
|---|
| Mol. Mass. | 543.5988 |
|---|
| SMILES | CN1CCN(CCCN([C@@H]2CC[C@@]3(C[C@H]23)c2ccc(cc2)C#N)C(=O)Nc2ccc(F)c(c2)C(F)(F)F)CC1 |
|---|
| Structure | 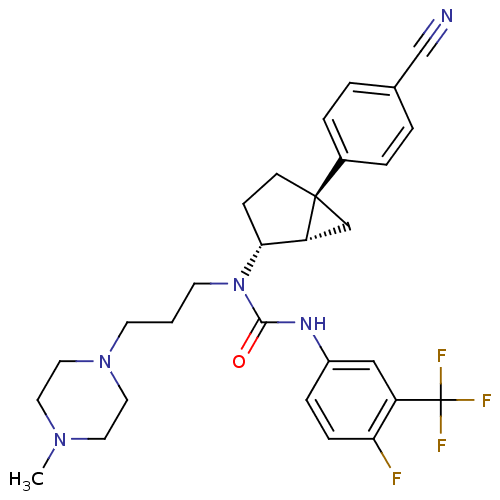
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hadden, M; Deering, DM; Henderson, AJ; Surman, MD; Luche, M; Khmelnitsky, Y; Vickers, S; Viggers, J; Cheetham, S; Guzzo, PR Synthesis and SAR of 4-aryl-1-(indazol-5-yl)pyridin-2(1H)ones as MCH-1 antagonists for the treatment of obesity. Bioorg Med Chem Lett20:7020-3 (2010) [PubMed] Article
Hadden, M; Deering, DM; Henderson, AJ; Surman, MD; Luche, M; Khmelnitsky, Y; Vickers, S; Viggers, J; Cheetham, S; Guzzo, PR Synthesis and SAR of 4-aryl-1-(indazol-5-yl)pyridin-2(1H)ones as MCH-1 antagonists for the treatment of obesity. Bioorg Med Chem Lett20:7020-3 (2010) [PubMed] Article