| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sphingosine 1-phosphate receptor 5 |
|---|
| Ligand | BDBM50335514 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_702156 (CHEMBL1655493) |
|---|
| EC50 | 29±n/a nM |
|---|
| Citation |  Cee, VJ; Frohn, M; Lanman, BA; Golden, J; Muller, K; Neira, S; Pickrell, A; Arnett, H; Buys, J; Gore, A; Fiorino, M; Horner, M; Itano, A; Lee, MR; McElvain, M; Middleton, S; Schrag, M; Rivenzon-Segal, D; Vargas, HM; Xu, H; Xu, Y; Zhang, X; Siu, J; Wong, M Discovery of AMG 369, a Thiazolo[5,4-b]pyridine Agonist of S1P1 and S1P5 ACS Med Chem Lett2:107-112 (2011) [PubMed] Article Cee, VJ; Frohn, M; Lanman, BA; Golden, J; Muller, K; Neira, S; Pickrell, A; Arnett, H; Buys, J; Gore, A; Fiorino, M; Horner, M; Itano, A; Lee, MR; McElvain, M; Middleton, S; Schrag, M; Rivenzon-Segal, D; Vargas, HM; Xu, H; Xu, Y; Zhang, X; Siu, J; Wong, M Discovery of AMG 369, a Thiazolo[5,4-b]pyridine Agonist of S1P1 and S1P5 ACS Med Chem Lett2:107-112 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sphingosine 1-phosphate receptor 5 |
|---|
| Name: | Sphingosine 1-phosphate receptor 5 |
|---|
| Synonyms: | EDG8 | Endothelial differentiation sphingolipid G-protein-coupled receptor 8 | S1P5 | S1PR5 | S1PR5_HUMAN | Sphingosine 1-phosphate receptor | Sphingosine 1-phosphate receptor Edg-8 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 41796.42 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 398 |
|---|
| Sequence: | MESGLLRPAPVSEVIVLHYNYTGKLRGARYQPGAGLRADAVVCLAVCAFIVLENLAVLLV
LGRHPRFHAPMFLLLGSLTLSDLLAGAAYAANILLSGPLTLKLSPALWFAREGGVFVALT
ASVLSLLAIALERSLTMARRGPAPVSSRGRTLAMAAAAWGVSLLLGLLPALGWNCLGRLD
ACSTVLPLYAKAYVLFCVLAFVGILAAICALYARIYCQVRANARRLPARPGTAGTTSTRA
RRKPRSLALLRTLSVVLLAFVACWGPLFLLLLLDVACPARTCPVLLQADPFLGLAMANSL
LNPIIYTLTNRDLRHALLRLVCCGRHSCGRDPSGSQQSASAAEASGGLRRCLPPGLDGSF
SGSERSSPQRDGLDTSGSTGSPGAPTAARTLVSEPAAD
|
|
|
|---|
| BDBM50335514 |
|---|
| n/a |
|---|
| Name | BDBM50335514 |
|---|
| Synonyms: | 1-((3-fluoro-4-(5-(1-phenylcyclopropyl)thiazolo[5,4-b]pyridin-2-yl)phenyl)methyl)azetidine-3-carboxylic acid | CHEMBL1651861 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H22FN3O2S |
|---|
| Mol. Mass. | 459.535 |
|---|
| SMILES | OC(=O)C1CN(Cc2ccc(-c3nc4ccc(nc4s3)C3(CC3)c3ccccc3)c(F)c2)C1 |
|---|
| Structure | 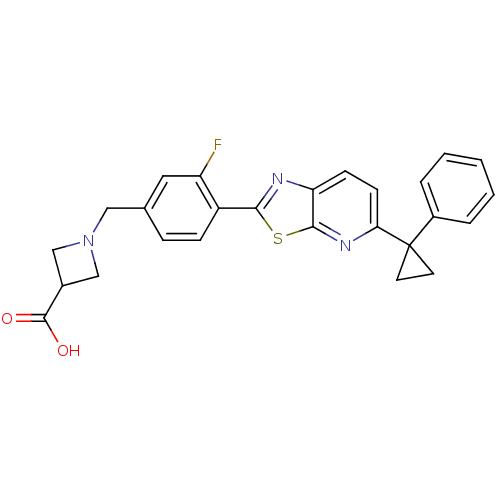
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cee, VJ; Frohn, M; Lanman, BA; Golden, J; Muller, K; Neira, S; Pickrell, A; Arnett, H; Buys, J; Gore, A; Fiorino, M; Horner, M; Itano, A; Lee, MR; McElvain, M; Middleton, S; Schrag, M; Rivenzon-Segal, D; Vargas, HM; Xu, H; Xu, Y; Zhang, X; Siu, J; Wong, M Discovery of AMG 369, a Thiazolo[5,4-b]pyridine Agonist of S1P1 and S1P5 ACS Med Chem Lett2:107-112 (2011) [PubMed] Article
Cee, VJ; Frohn, M; Lanman, BA; Golden, J; Muller, K; Neira, S; Pickrell, A; Arnett, H; Buys, J; Gore, A; Fiorino, M; Horner, M; Itano, A; Lee, MR; McElvain, M; Middleton, S; Schrag, M; Rivenzon-Segal, D; Vargas, HM; Xu, H; Xu, Y; Zhang, X; Siu, J; Wong, M Discovery of AMG 369, a Thiazolo[5,4-b]pyridine Agonist of S1P1 and S1P5 ACS Med Chem Lett2:107-112 (2011) [PubMed] Article