| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50296980 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_748201 (CHEMBL1780282) |
|---|
| IC50 | 34000±n/a nM |
|---|
| Citation |  Zaghdane, H; Boyd, M; Colucci, J; Simard, D; Berthelette, C; Leblanc, Y; Wang, Z; Houle, R; Lévesque, JF; Molinaro, C; Hamel, M; Stocco, R; Sawyer, N; Sillaots, S; Gervais, F; Gallant, M New indole amide derivatives as potent CRTH2 receptor antagonists. Bioorg Med Chem Lett21:3471-4 (2011) [PubMed] Article Zaghdane, H; Boyd, M; Colucci, J; Simard, D; Berthelette, C; Leblanc, Y; Wang, Z; Houle, R; Lévesque, JF; Molinaro, C; Hamel, M; Stocco, R; Sawyer, N; Sillaots, S; Gervais, F; Gallant, M New indole amide derivatives as potent CRTH2 receptor antagonists. Bioorg Med Chem Lett21:3471-4 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50296980 |
|---|
| n/a |
|---|
| Name | BDBM50296980 |
|---|
| Synonyms: | (R)-2-(7-(4-fluoro-N-methylphenylsulfonamido)-6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)acetic acid | CHEMBL561132 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H21FN2O4S |
|---|
| Mol. Mass. | 416.466 |
|---|
| SMILES | CN([C@@H]1CCc2c(CC(O)=O)c3ccccc3n2C1)S(=O)(=O)c1ccc(F)cc1 |r| |
|---|
| Structure | 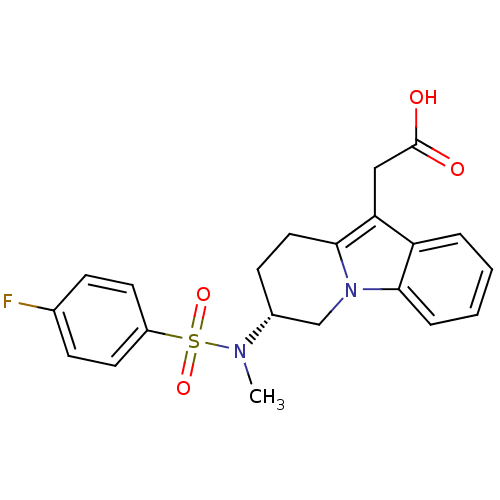
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zaghdane, H; Boyd, M; Colucci, J; Simard, D; Berthelette, C; Leblanc, Y; Wang, Z; Houle, R; Lévesque, JF; Molinaro, C; Hamel, M; Stocco, R; Sawyer, N; Sillaots, S; Gervais, F; Gallant, M New indole amide derivatives as potent CRTH2 receptor antagonists. Bioorg Med Chem Lett21:3471-4 (2011) [PubMed] Article
Zaghdane, H; Boyd, M; Colucci, J; Simard, D; Berthelette, C; Leblanc, Y; Wang, Z; Houle, R; Lévesque, JF; Molinaro, C; Hamel, M; Stocco, R; Sawyer, N; Sillaots, S; Gervais, F; Gallant, M New indole amide derivatives as potent CRTH2 receptor antagonists. Bioorg Med Chem Lett21:3471-4 (2011) [PubMed] Article