| Reaction Details |
|---|
 | Report a problem with these data |
| Target | MAP kinase-activated protein kinase 2 |
|---|
| Ligand | BDBM50344718 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_749048 (CHEMBL1780645) |
|---|
| EC50 | 8.3±n/a nM |
|---|
| Citation |  Kaptein, A; Oubrie, A; de Zwart, E; Hoogenboom, N; de Wit, J; van de Kar, B; van Hoek, M; Vogel, G; de Kimpe, V; Schultz-Fademrecht, C; Borsboom, J; van Zeeland, M; Versteegh, J; Kazemier, B; de Roos, J; Wijnands, F; Dulos, J; Jaeger, M; Leandro-Garcia, P; Barf, T Discovery of selective and orally available spiro-3-piperidyl ATP-competitive MK2 inhibitors. Bioorg Med Chem Lett21:3823-7 (2011) [PubMed] Article Kaptein, A; Oubrie, A; de Zwart, E; Hoogenboom, N; de Wit, J; van de Kar, B; van Hoek, M; Vogel, G; de Kimpe, V; Schultz-Fademrecht, C; Borsboom, J; van Zeeland, M; Versteegh, J; Kazemier, B; de Roos, J; Wijnands, F; Dulos, J; Jaeger, M; Leandro-Garcia, P; Barf, T Discovery of selective and orally available spiro-3-piperidyl ATP-competitive MK2 inhibitors. Bioorg Med Chem Lett21:3823-7 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| MAP kinase-activated protein kinase 2 |
|---|
| Name: | MAP kinase-activated protein kinase 2 |
|---|
| Synonyms: | MAP kinase-activated protein kinase 2 (MAPKAPK2) | MAP kinase-activated protein kinase 2 (MK2) | MAP kinase-activated protein kinase 2 (p38/MK2) | MAPK-Activated Protein Kinase 2 (MK2) | MAPK-activated protein kinase 2 | MAPK2_HUMAN | MAPKAP kinase 2 | MAPKAPK-2 | MAPKAPK2 | MK2 |
|---|
| Type: | Serine/threonine-protein kinase |
|---|
| Mol. Mass.: | 45579.87 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49137 |
|---|
| Residue: | 400 |
|---|
| Sequence: | MLSNSQGQSPPVPFPAPAPPPQPPTPALPHPPAQPPPPPPQQFPQFHVKSGLQIKKNAII
DDYKVTSQVLGLGINGKVLQIFNKRTQEKFALKMLQDCPKARREVELHWRASQCPHIVRI
VDVYENLYAGRKCLLIVMECLDGGELFSRIQDRGDQAFTEREASEIMKSIGEAIQYLHSI
NIAHRDVKPENLLYTSKRPNAILKLTDFGFAKETTSHNSLTTPCYTPYYVAPEVLGPEKY
DKSCDMWSLGVIMYILLCGYPPFYSNHGLAISPGMKTRIRMGQYEFPNPEWSEVSEEVKM
LIRNLLKTEPTQRMTITEFMNHPWIMQSTKVPQTPLHTSRVLKEDKERWEDVKEEMTSAL
ATMRVDYEQIKIKKIEDASNPLLLKRRKKARALEAAALAH
|
|
|
|---|
| BDBM50344718 |
|---|
| n/a |
|---|
| Name | BDBM50344718 |
|---|
| Synonyms: | 2'-(2-(3-fluorophenyl)pyrimidin-4-yl)-5',6'-dihydrospiro[piperidine-4,7'-pyrrolo[3,2-c]pyridin]-4'(1'H)-one | CHEMBL1779350 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H20FN5O |
|---|
| Mol. Mass. | 377.4148 |
|---|
| SMILES | Fc1cccc(c1)-c1nccc(n1)-c1cc2c([nH]1)C1(CCNCC1)CNC2=O |
|---|
| Structure | 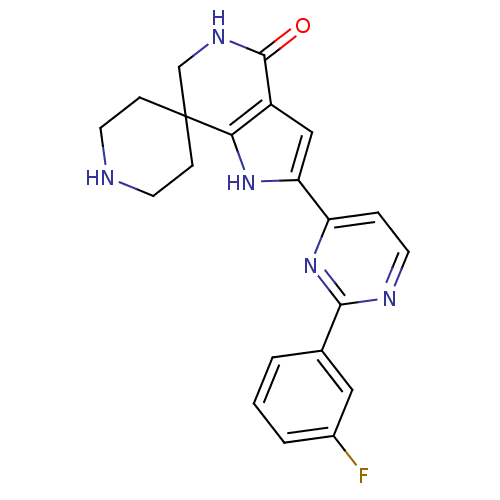
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kaptein, A; Oubrie, A; de Zwart, E; Hoogenboom, N; de Wit, J; van de Kar, B; van Hoek, M; Vogel, G; de Kimpe, V; Schultz-Fademrecht, C; Borsboom, J; van Zeeland, M; Versteegh, J; Kazemier, B; de Roos, J; Wijnands, F; Dulos, J; Jaeger, M; Leandro-Garcia, P; Barf, T Discovery of selective and orally available spiro-3-piperidyl ATP-competitive MK2 inhibitors. Bioorg Med Chem Lett21:3823-7 (2011) [PubMed] Article
Kaptein, A; Oubrie, A; de Zwart, E; Hoogenboom, N; de Wit, J; van de Kar, B; van Hoek, M; Vogel, G; de Kimpe, V; Schultz-Fademrecht, C; Borsboom, J; van Zeeland, M; Versteegh, J; Kazemier, B; de Roos, J; Wijnands, F; Dulos, J; Jaeger, M; Leandro-Garcia, P; Barf, T Discovery of selective and orally available spiro-3-piperidyl ATP-competitive MK2 inhibitors. Bioorg Med Chem Lett21:3823-7 (2011) [PubMed] Article