

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50118719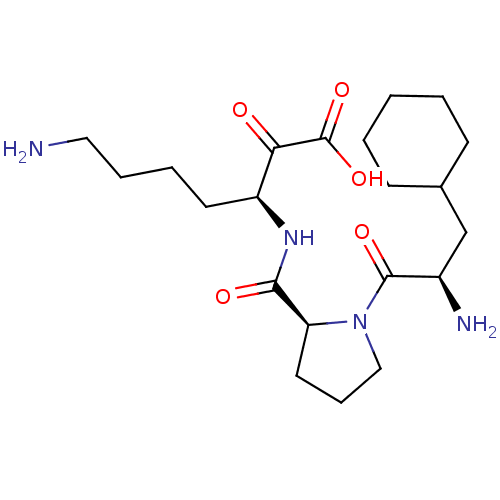 (7-Amino-3-{[1-(2-amino-3-cyclohexyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118730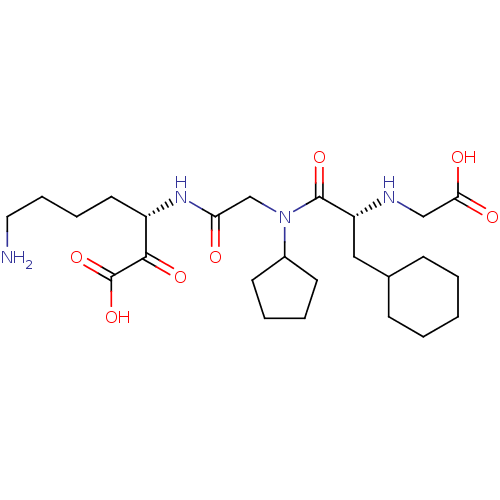 (7-Amino-3-(2-{[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118728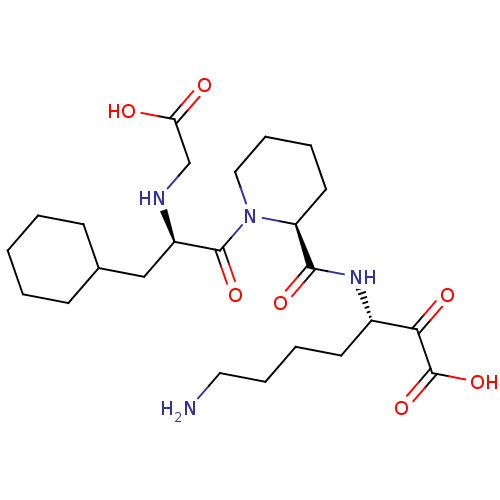 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118739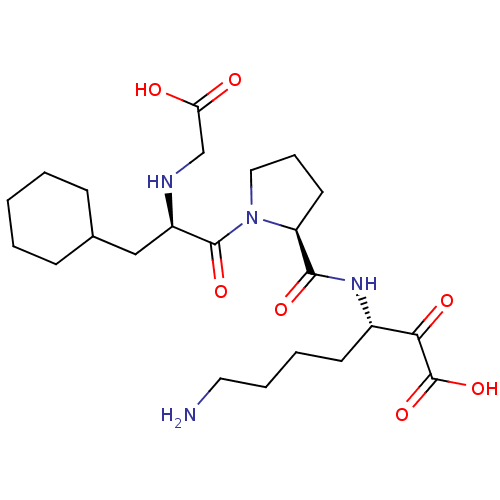 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118732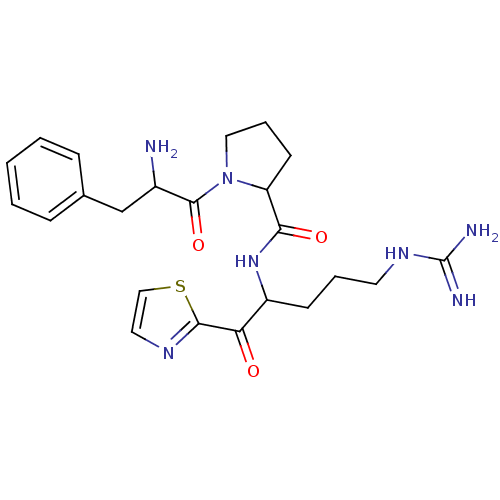 (1-(2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118729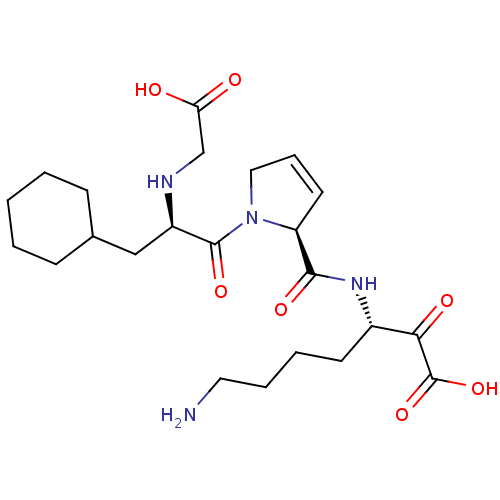 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118731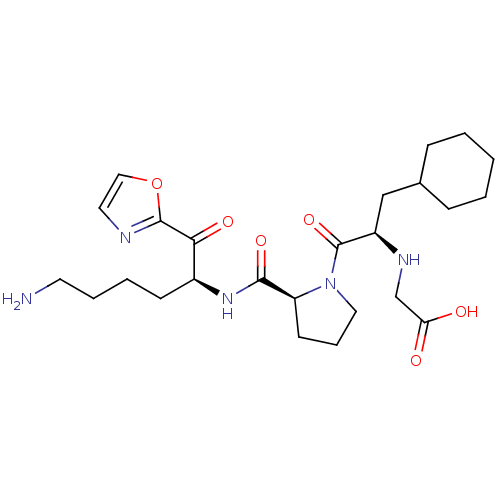 ((2-{2-[5-Amino-1-(oxazole-2-carbonyl)-pentylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118735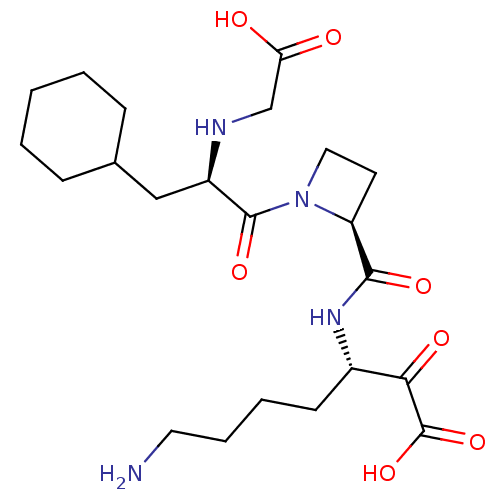 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118738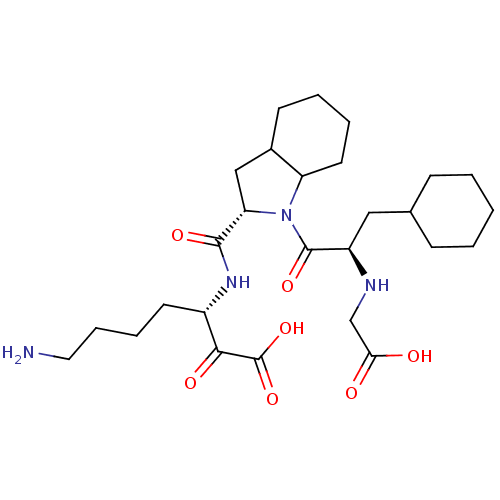 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118718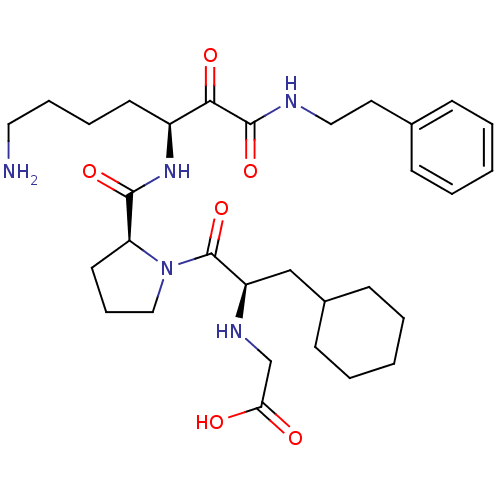 (CHEMBL343804 | {2-[2-(5-Amino-1-phenethylaminooxal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118723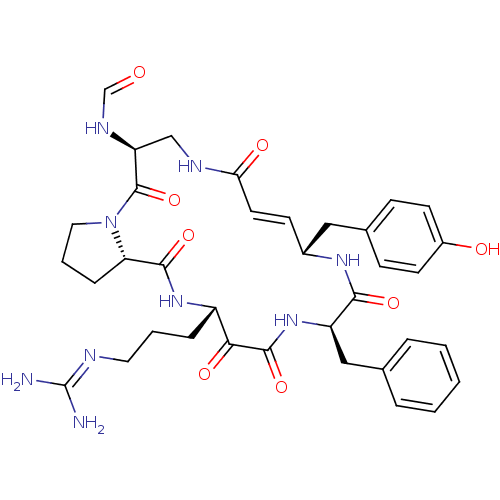 (CHEMBL342672 | CYCLOTHEONAMIDE A | N-[14-Benzyl-18...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118727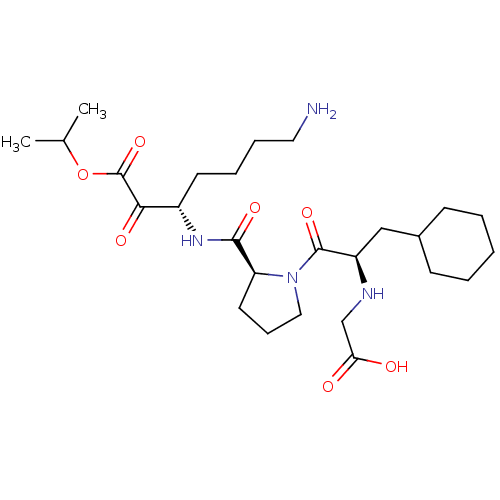 (2-((R)-1-((S)-2-(((S)-7-amino-1-isopropoxy-1,2-dio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118720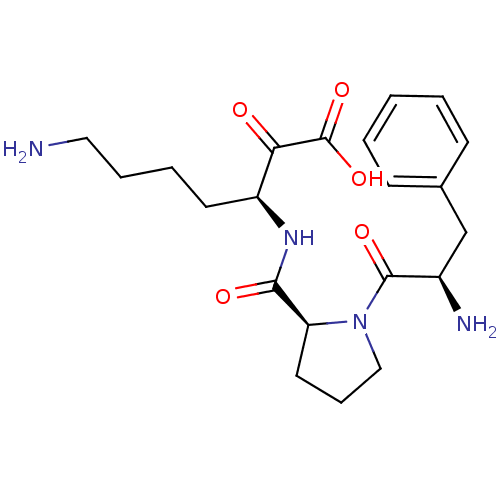 (7-Amino-3-{[1-(2-amino-3-phenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM29388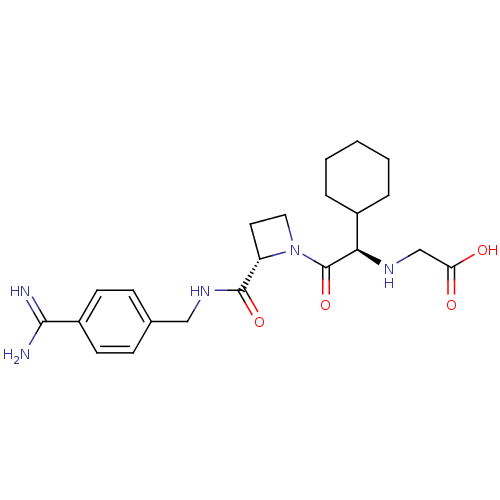 (Exanta | Melagatran | US11584714, Compound 999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118717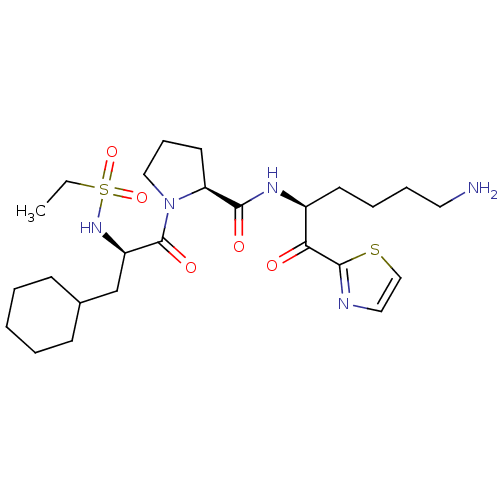 ((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118737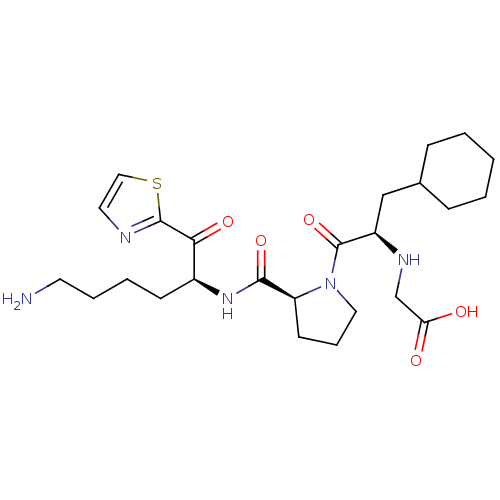 ((2-{2-[5-Amino-1-(thiazole-2-carbonyl)-pentylcarba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118721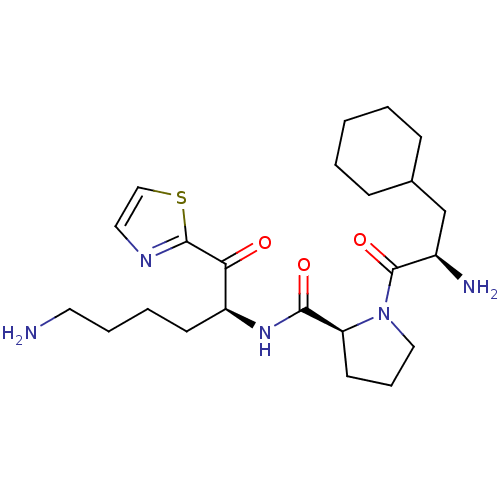 ((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118722 (2-Amino-N-{[5-amino-1-(thiazole-2-carbonyl)-pentyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184539 ((2S)-2-(6-butyl-2-(4-(4-(trifluoromethyl)phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118725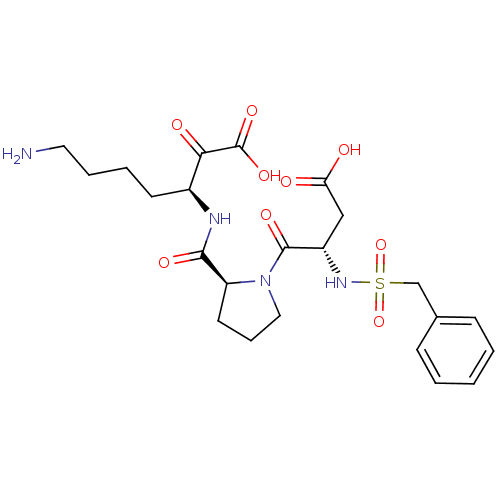 (7-Amino-3-{[1-(3-carboxy-2-phenylmethanesulfonylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184530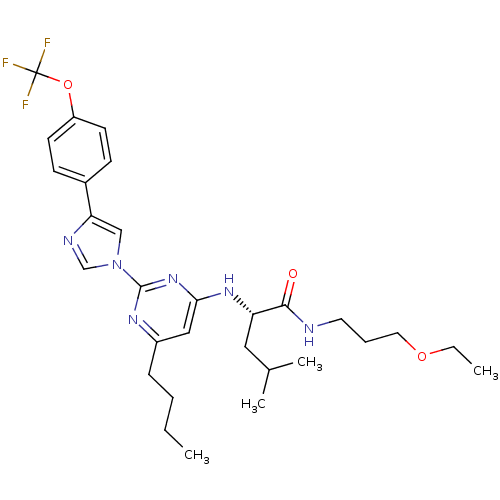 ((2S)-2-(6-butyl-2-(4-(4-(trifluoromethoxy)phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038001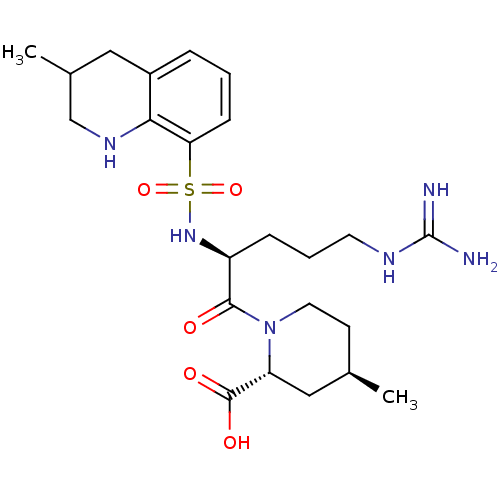 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184531 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184518 ((2S)-2-(6-butyl-2-(4-(4-chlorophenyl)-1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118734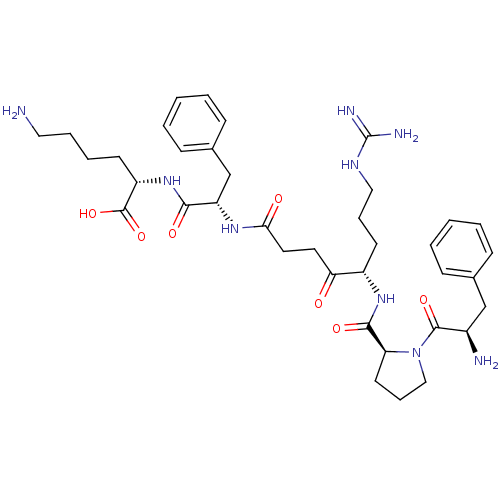 (6-Amino-2-[2-(5-{[1-(2-amino-3-phenyl-propionyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184527 ((2S)-2-(6-butyl-2-(4-(4-hydroxyphenyl)-1H-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184540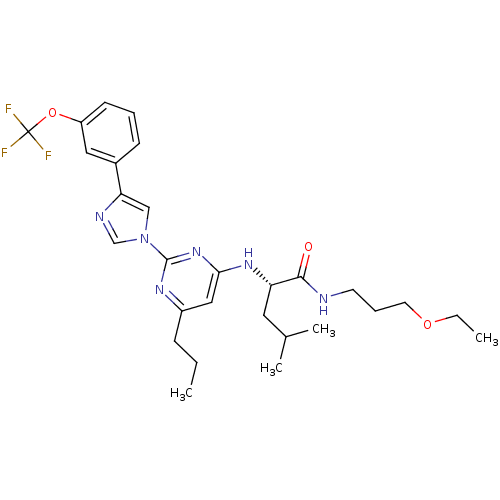 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184536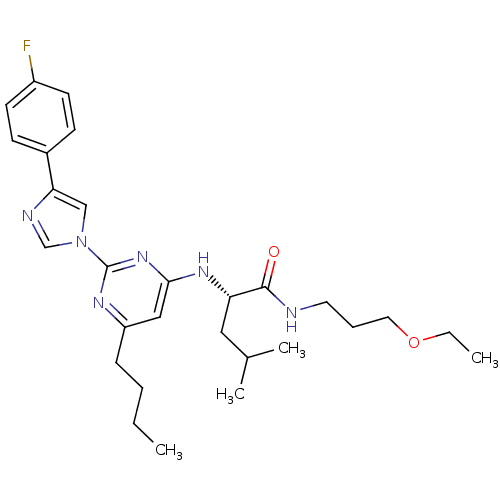 ((2S)-2-(6-butyl-2-(4-(4-fluorophenyl)-1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184535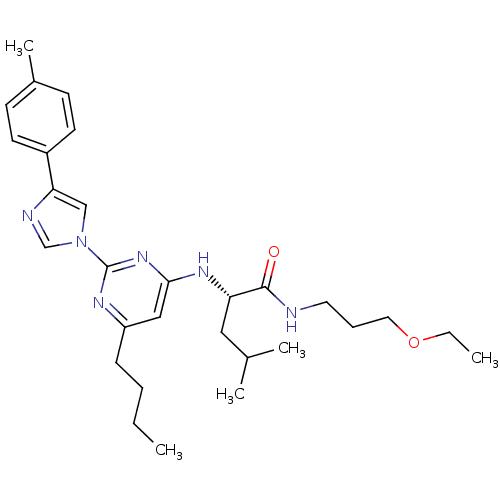 ((2S)-2-(6-butyl-2-(4-p-tolyl-1H-imidazol-1-yl)pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184521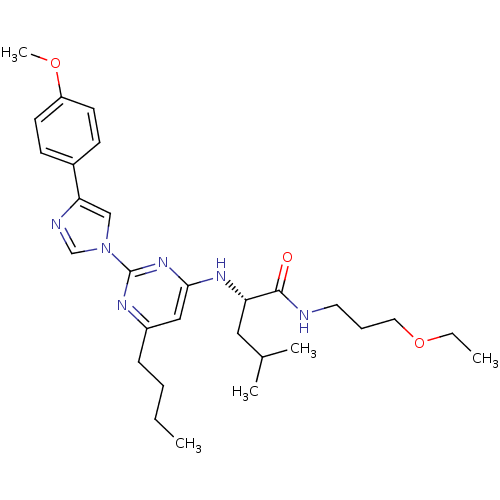 ((2S)-2-(6-butyl-2-(4-(4-methoxyphenyl)-1H-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184523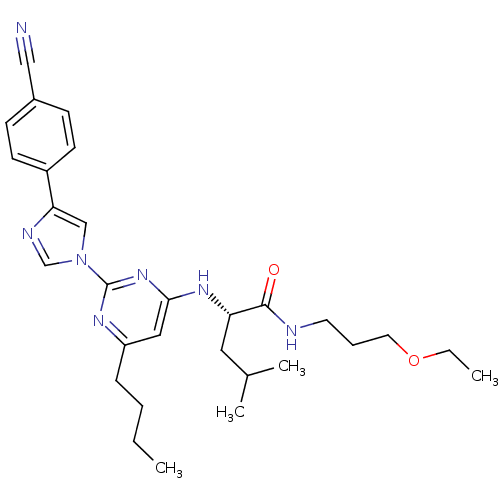 ((2S)-2-(6-butyl-2-(4-(4-cyanophenyl)-1H-imidazol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184517 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-methyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184537 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184516 ((2S)-N-(3-ethoxypropyl)-2-(6-ethyl-2-(4-(4-(triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184524 ((2S)-2-(2-(1H-imidazol-1-yl)-6-(octylthio)pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184532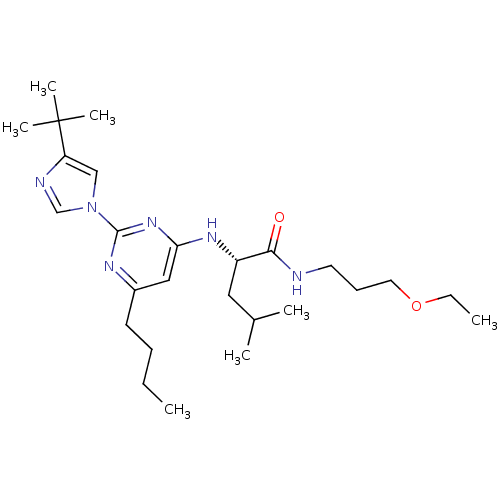 ((2S)-2-(6-butyl-2-(4-tert-butyl-1H-imidazol-1-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184541 ((2S)-2-(6-butyl-2-(4-methyl-1H-imidazol-1-yl)pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184542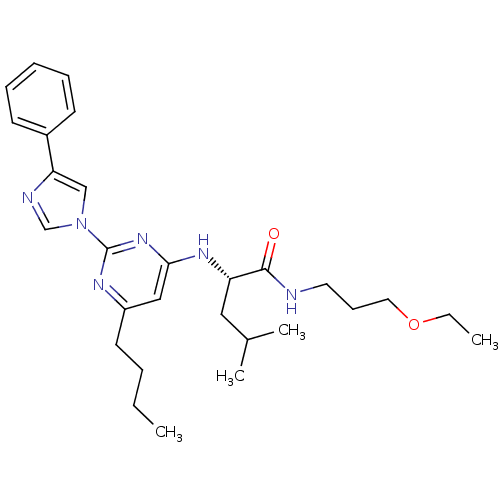 ((2S)-2-(6-butyl-2-(4-phenyl-1H-imidazol-1-yl)pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184519 ((2S)-2-(2-(1H-imidazol-1-yl)-6-octylpyrimidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118724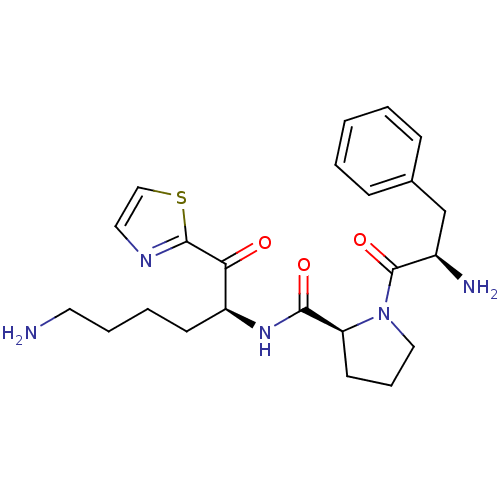 ((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184526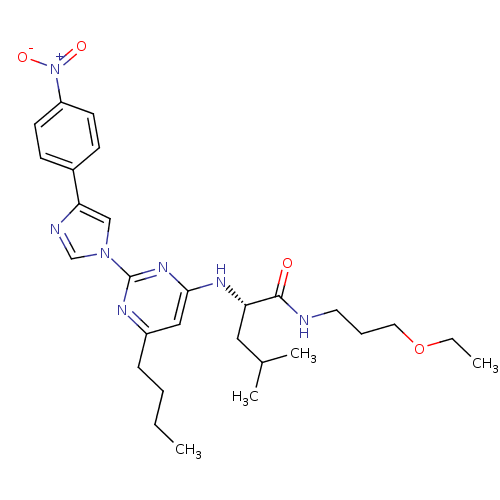 ((2S)-2-(6-butyl-2-(4-(4-nitrophenyl)-1H-imidazol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184529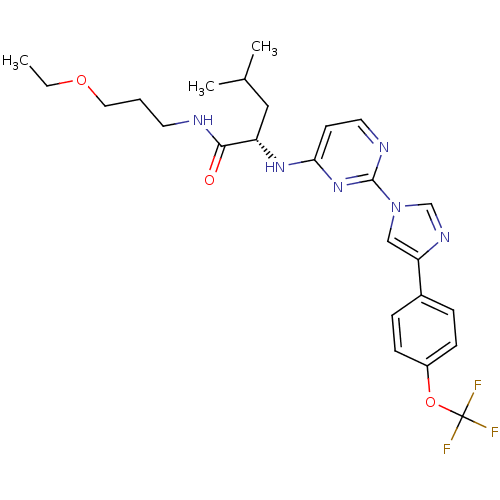 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(2-(4-(4-(trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118716 ((S)-1-(3,3-diphenyl-propionyl)-pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184538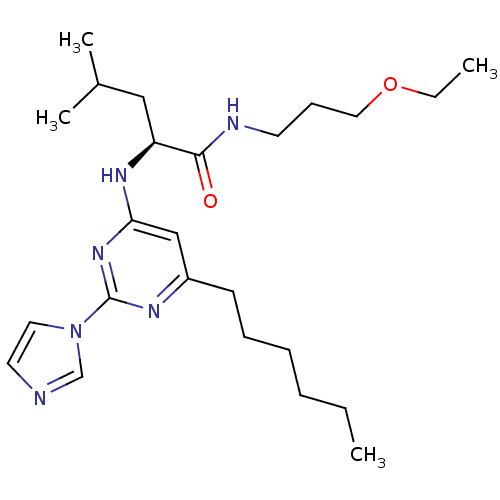 ((2S)-N-(3-ethoxypropyl)-2-(6-hexyl-2-(1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118715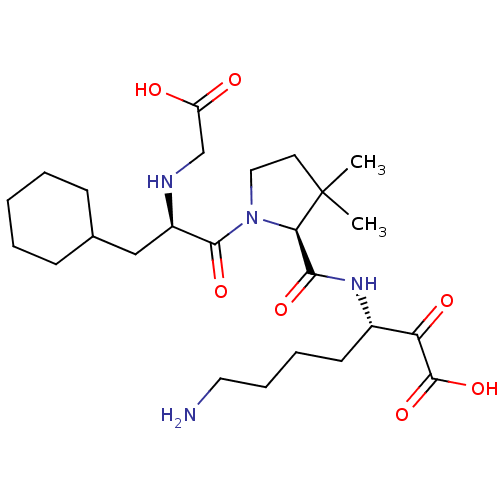 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184528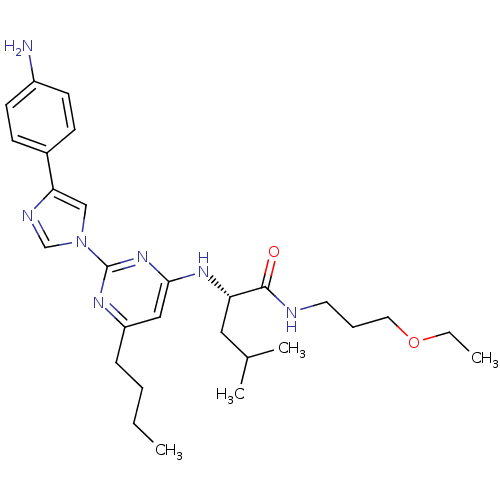 ((2S)-2-(2-(4-(4-aminophenyl)-1H-imidazol-1-yl)-6-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184525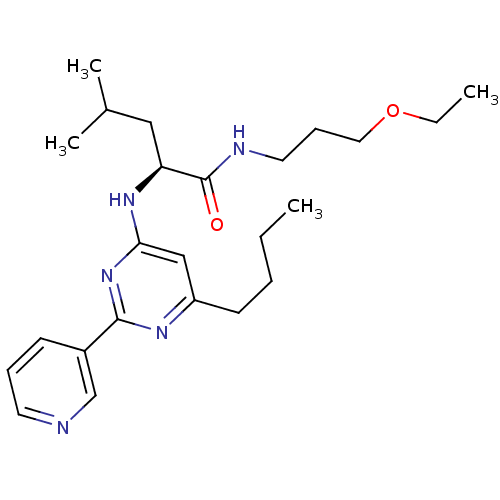 ((2S)-2-(6-butyl-2-(pyridin-3-yl)pyrimidin-4-ylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118733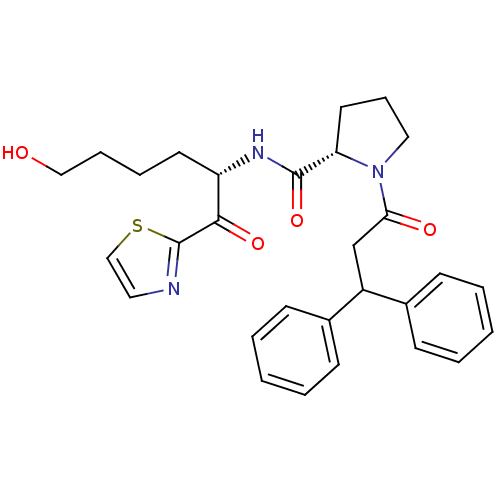 (1-(3,3-Diphenyl-propionyl)-pyrrolidine-2-carboxyli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118726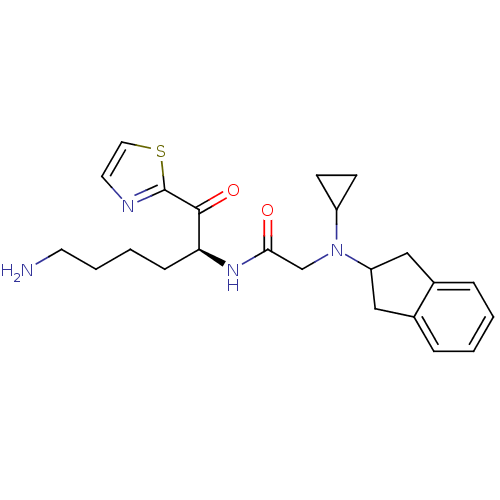 (CHEMBL334701 | N-[5-Amino-1-(thiazole-2-carbonyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118736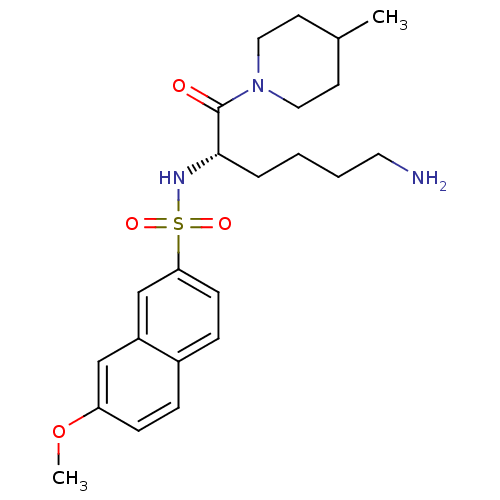 (7-Methoxy-naphthalene-2-sulfonic acid [5-amino-1-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |