| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha |
|---|
| Ligand | BDBM50346961 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_753293 (CHEMBL1799504) |
|---|
| IC50 | 1110±n/a nM |
|---|
| Citation |  Liu, Q; Wang, J; Kang, SA; Thoreen, CC; Hur, W; Choi, HG; Waller, DL; Sim, T; Sabatini, DM; Gray, NS Discovery and optimization of potent and selective benzonaphthyridinone analogs as small molecule mTOR inhibitors with improved mouse microsome stability. Bioorg Med Chem Lett21:4036-40 (2011) [PubMed] Article Liu, Q; Wang, J; Kang, SA; Thoreen, CC; Hur, W; Choi, HG; Waller, DL; Sim, T; Sabatini, DM; Gray, NS Discovery and optimization of potent and selective benzonaphthyridinone analogs as small molecule mTOR inhibitors with improved mouse microsome stability. Bioorg Med Chem Lett21:4036-40 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha |
|---|
| Name: | Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha |
|---|
| Synonyms: | P3C2A_HUMAN | PI3K-C2-alpha | PIK3C2A | Phosphatidylinositol 4-phosphate 3-kinase C2 alpha (PIK3C2A) | Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing subunit alpha | Phosphoinositide 3-kinase-C2-alpha | PtdIns-3-kinase C2 subunit alpha |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 190704.50 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O00443 |
|---|
| Residue: | 1686 |
|---|
| Sequence: | MAQISSNSGFKECPSSHPEPTRAKDVDKEEALQMEAEALAKLQKDRQVTDNQRGFELSSS
TRKKAQVYNKQDYDLMVFPESDSQKRALDIDVEKLTQAELEKLLLDDSFETKKTPVLPVT
PILSPSFSAQLYFRPTIQRGQWPPGLPGPSTYALPSIYPSTYSKQAAFQNGFNPRMPTFP
STEPIYLSLPGQSPYFSYPLTPATPFHPQGSLPIYRPVVSTDMAKLFDKIASTSEFLKNG
KARTDLEITDSKVSNLQVSPKSEDISKFDWLDLDPLSKPKVDNVEVLDHEEEKNVSSLLA
KDPWDAVLLEERSTANCHLERKVNGKSLSVATVTRSQSLNIRTTQLAKAQGHISQKDPNG
TSSLPTGSSLLQEVEVQNEEMAAFCRSITKLKTKFPYTNHRTNPGYLLSPVTAQRNICGE
NASVKVSIDIEGFQLPVTFTCDVSSTVEIIIMQALCWVHDDLNQVDVGSYVLKVCGQEEV
LQNNHCLGSHEHIQNCRKWDTEIRLQLLTFSAMCQNLARTAEDDETPVDLNKHLYQIEKP
CKEAMTRHPVEELLDSYHNQVELALQIENQHRAVDQVIKAVRKICSALDGVETLAITESV
KKLKRAVNLPRSKTADVTSLFGGEDTSRSSTRGSLNPENPVQVSINQLTAAIYDLLRLHA
NSGRSPTDCAQSSKSVKEAWTTTEQLQFTIFAAHGISSNWVSNYEKYYLICSLSHNGKDL
FKPIQSKKVGTYKNFFYLIKWDELIIFPIQISQLPLESVLHLTLFGILNQSSGSSPDSNK
QRKGPEALGKVSLPLFDFKRFLTCGTKLLYLWTSSHTNSVPGTVTKKGYVMERIVLQVDF
PSPAFDIIYTTPQVDRSIIQQHNLETLENDIKGKLLDILHKDSSLGLSKEDKAFLWEKRY
YCFKHPNCLPKILASAPNWKWVNLAKTYSLLHQWPALYPLIALELLDSKFADQEVRSLAV
TWIEAISDDELTDLLPQFVQALKYEIYLNSSLVQFLLSRALGNIQIAHNLYWLLKDALHD
VQFSTRYEHVLGALLSVGGKRLREELLKQTKLVQLLGGVAEKVRQASGSARQVVLQRSME
RVQSFFQKNKCRLPLKPSLVAKELNIKSCSFFSSNAVPLKVTMVNADPMGEEINVMFKVG
EDLRQDMLALQMIKIMDKIWLKEGLDLRMVIFKCLSTGRDRGMVELVPASDTLRKIQVEY
GVTGSFKDKPLAEWLRKYNPSEEEYEKASENFIYSCAGCCVATYVLGICDRHNDNIMLRS
TGHMFHIDFGKFLGHAQMFGSFKRDRAPFVLTSDMAYVINGGEKPTIRFQLFVDLCCQAY
NLIRKQTNLFLNLLSLMIPSGLPELTSIQDLKYVRDALQPQTTDAEATIFFTRLIESSLG
SIATKFNFFIHNLAQLRFSGLPSNDEPILSFSPKTYSFRQDGRIKEVSVFTYHKKYNPDK
HYIYVVRILREGQIEPSFVFRTFDEFQELHNKLSIIFPLWKLPGFPNRMVLGRTHIKDVA
AKRKIELNSYLQSLMNASTDVAECDLVCTFFHPLLRDEKAEGIARSADAGSFSPTPGQIG
GAVKLSISYRNGTLFIMVMHIKDLVTEDGADPNPYVKTYLLPDNHKTSKRKTKISRKTRN
PTFNEMLVYSGYSKETLRQRELQLSVLSAESLRENFFLGGVTLPLKDFNLSKETVKWYQL
TAATYL
|
|
|
|---|
| BDBM50346961 |
|---|
| n/a |
|---|
| Name | BDBM50346961 |
|---|
| Synonyms: | CHEMBL1795875 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C41H32F3N5O2 |
|---|
| Mol. Mass. | 683.7203 |
|---|
| SMILES | CN1CCC(CC1)NC(=O)c1ccc(cc1)-c1ccc(cc1C(F)(F)F)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2ccccc2c1 |
|---|
| Structure | 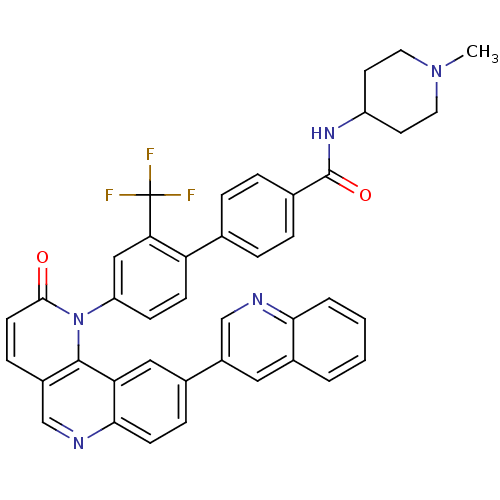
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, Q; Wang, J; Kang, SA; Thoreen, CC; Hur, W; Choi, HG; Waller, DL; Sim, T; Sabatini, DM; Gray, NS Discovery and optimization of potent and selective benzonaphthyridinone analogs as small molecule mTOR inhibitors with improved mouse microsome stability. Bioorg Med Chem Lett21:4036-40 (2011) [PubMed] Article
Liu, Q; Wang, J; Kang, SA; Thoreen, CC; Hur, W; Choi, HG; Waller, DL; Sim, T; Sabatini, DM; Gray, NS Discovery and optimization of potent and selective benzonaphthyridinone analogs as small molecule mTOR inhibitors with improved mouse microsome stability. Bioorg Med Chem Lett21:4036-40 (2011) [PubMed] Article