| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Xaa-Pro aminopeptidase 2 |
|---|
| Ligand | BDBM50351402 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_764499 (CHEMBL1821051) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Nishio, Y; Kimura, H; Sawada, N; Sugaru, E; Horiguchi, M; Ono, M; Furuta, Y; Sakai, M; Masui, Y; Otani, M; Hashizuka, T; Honda, Y; Deguchi, J; Nakagawa, T; Nakahira, H 2-({6-[(3R)-3-amino-3-methylpiperidine-1-yl]-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrrolo[3,2-d]pyrimidine-5-yl}methyl)-4-fluorobenzonitrile (DSR-12727): a potent, orally active dipeptidyl peptidase IV inhibitor without mechanism-based inactivation of CYP3A. Bioorg Med Chem19:5490-9 (2011) [PubMed] Article Nishio, Y; Kimura, H; Sawada, N; Sugaru, E; Horiguchi, M; Ono, M; Furuta, Y; Sakai, M; Masui, Y; Otani, M; Hashizuka, T; Honda, Y; Deguchi, J; Nakagawa, T; Nakahira, H 2-({6-[(3R)-3-amino-3-methylpiperidine-1-yl]-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrrolo[3,2-d]pyrimidine-5-yl}methyl)-4-fluorobenzonitrile (DSR-12727): a potent, orally active dipeptidyl peptidase IV inhibitor without mechanism-based inactivation of CYP3A. Bioorg Med Chem19:5490-9 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Xaa-Pro aminopeptidase 2 |
|---|
| Name: | Xaa-Pro aminopeptidase 2 |
|---|
| Synonyms: | Membrane-bound aminopeptidase P (APP2) | XPNPEP2 | XPP2_HUMAN | Xaa-Pro aminopeptidase 2 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 75618.88 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O43895 |
|---|
| Residue: | 674 |
|---|
| Sequence: | MARAHWGCCPWLVLLCACAWGHTKPVDLGGQDVRNCSTNPPYLPVTVVNTTMSLTALRQQ
MQTQNLSAYIIPGTDAHMNEYIGQHDERRAWITGFTGSAGTAVVTMKKAAVWTDSRYWTQ
AERQMDCNWELHKEVGTTPIVTWLLTEIPAGGRVGFDPFLLSIDTWESYDLALQGSNRQL
VSITTNLVDLVWGSERPPVPNQPIYALQEAFTGSTWQEKVSGVRSQMQKHQKVPTAVLLS
ALEETAWLFNLRASDIPYNPFFYSYTLLTDSSIRLFANKSRFSSETLSYLNSSCTGPMCV
QIEDYSQVRDSIQAYSLGDVRIWIGTSYTMYGIYEMIPKEKLVTDTYSPVMMTKAVKNSK
EQALLKASHVRDAVAVIRYLVWLEKNVPKGTVDEFSGAEIVDKFRGEEQFSSGPSFETIS
ASGLNAALAHYSPTKELNRKLSSDEMYLLDSGGQYWDGTTDITRTVHWGTPSAFQKEAYT
RVLIGNIDLSRLIFPAATSGRMVEAFARRALWDAGLNYGHGTGHGIGNFLCVHEWPVGFQ
SNNIAMAKGMFTSIEPGYYKDGEFGIRLEDVALVVEAKTKYPGSYLTFEVVSFVPYDRNL
IDVSLLSPEHLQYLNRYYQTIREKVGPELQRRQLLEEFEWLQQHTEPLAARAPDTASWAS
VLVVSTLAILGWSV
|
|
|
|---|
| BDBM50351402 |
|---|
| n/a |
|---|
| Name | BDBM50351402 |
|---|
| Synonyms: | CHEMBL1819090 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H25FN6O2 |
|---|
| Mol. Mass. | 424.4713 |
|---|
| SMILES | Cn1c2cc(N3CCC[C@@](C)(N)C3)n(Cc3cc(F)ccc3C#N)c2c(=O)n(C)c1=O |r| |
|---|
| Structure | 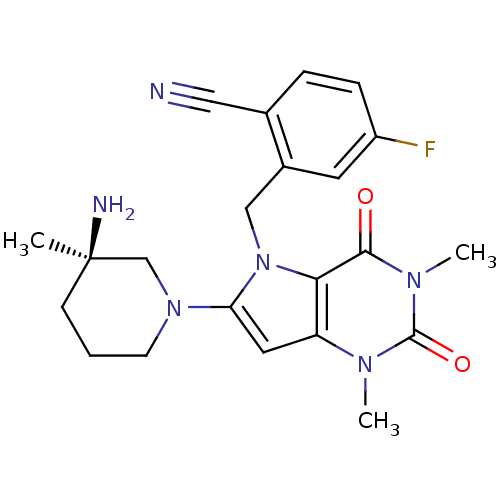
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nishio, Y; Kimura, H; Sawada, N; Sugaru, E; Horiguchi, M; Ono, M; Furuta, Y; Sakai, M; Masui, Y; Otani, M; Hashizuka, T; Honda, Y; Deguchi, J; Nakagawa, T; Nakahira, H 2-({6-[(3R)-3-amino-3-methylpiperidine-1-yl]-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrrolo[3,2-d]pyrimidine-5-yl}methyl)-4-fluorobenzonitrile (DSR-12727): a potent, orally active dipeptidyl peptidase IV inhibitor without mechanism-based inactivation of CYP3A. Bioorg Med Chem19:5490-9 (2011) [PubMed] Article
Nishio, Y; Kimura, H; Sawada, N; Sugaru, E; Horiguchi, M; Ono, M; Furuta, Y; Sakai, M; Masui, Y; Otani, M; Hashizuka, T; Honda, Y; Deguchi, J; Nakagawa, T; Nakahira, H 2-({6-[(3R)-3-amino-3-methylpiperidine-1-yl]-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrrolo[3,2-d]pyrimidine-5-yl}methyl)-4-fluorobenzonitrile (DSR-12727): a potent, orally active dipeptidyl peptidase IV inhibitor without mechanism-based inactivation of CYP3A. Bioorg Med Chem19:5490-9 (2011) [PubMed] Article