| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glutamate receptor ionotropic, NMDA 1 |
|---|
| Ligand | BDBM50069490 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_140302 |
|---|
| IC50 | 40±n/a nM |
|---|
| Citation |  Acklin, P; Allgeier, H; Auberson, YP; Bischoff, S; Ofner, S; Sauer, D; Schmutz, M 5-Aminomethylquinoxaline-2,3-diones, Part III: Arylamide derivatives as highly potent and selective glycine-site NMDA receptor antagonists. Bioorg Med Chem Lett8:493-8 (1999) [PubMed] Acklin, P; Allgeier, H; Auberson, YP; Bischoff, S; Ofner, S; Sauer, D; Schmutz, M 5-Aminomethylquinoxaline-2,3-diones, Part III: Arylamide derivatives as highly potent and selective glycine-site NMDA receptor antagonists. Bioorg Med Chem Lett8:493-8 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glutamate receptor ionotropic, NMDA 1 |
|---|
| Name: | Glutamate receptor ionotropic, NMDA 1 |
|---|
| Synonyms: | Glutamate (NMDA) receptor subunit zeta 1 | Glutamate [NMDA] receptor subunit zeta-1 | Glutamate-NMDA-Channel | Glutamate-NMDA-MK801 | Glutamate-NMDA-Polyamine | Grin1 | NMDA | NMDZ1_RAT | Nmdar1 | phencyclidine |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 105533.40 |
|---|
| Organism: | RAT |
|---|
| Description: | P35439 |
|---|
| Residue: | 938 |
|---|
| Sequence: | MSTMHLLTFALLFSCSFARAACDPKIVNIGAVLSTRKHEQMFREAVNQANKRHGSWKIQL
NATSVTHKPNAIQMALSVCEDLISSQVYAILVSHPPTPNDHFTPTPVSYTAGFYRIPVLG
LTTRMSIYSDKSIHLSFLRTVPPYSHQSSVWFEMMRVYNWNHIILLVSDDHEGRAAQKRL
ETLLEERESKAEKVLQFDPGTKNVTALLMEARELEARVIILSASEDDAATVYRAAAMLNM
TGSGYVWLVGEREISGNALRYAPDGIIGLQLINGKNESAHISDAVGVVAQAVHELLEKEN
ITDPPRGCVGNTNIWKTGPLFKRVLMSSKYADGVTGRVEFNEDGDRKFANYSIMNLQNRK
LVQVGIYNGTHVIPNDRKIIWPGGETEKPRGYQMSTRLKIVTIHQEPFVYVKPTMSDGTC
KEEFTVNGDPVKKVICTGPNDTSPGSPRHTVPQCCYGFCIDLLIKLARTMNFTYEVHLVA
DGKFGTQERVNNSNKKEWNGMMGELLSGQADMIVAPLTINNERAQYIEFSKPFKYQGLTI
LVKKEIPRSTLDSFMQPFQSTLWLLVGLSVHVVAVMLYLLDRFSPFGRFKVNSEEEEEDA
LTLSSAMWFSWGVLLNSGIGEGAPRSFSARILGMVWAGFAMIIVASYTANLAAFLVLDRP
EERITGINDPRLRNPSDKFIYATVKQSSVDIYFRRQVELSTMYRHMEKHNYESAAEAIQA
VRDNKLHAFIWDSAVLEFEASQKCDLVTTGELFFRSGFGIGMRKDSPWKQNVSLSILKSH
ENGFMEDLDKTWVRYQECDSRSNAPATLTFENMAGVFMLVAGGIVAGIFLIFIEIAYKRH
KDARRKQMQLAFAAVNVWRKNLQDRKSGRAEPDPKKKATFRAITSTLASSFKRRRSSKDT
STGGGRGALQNQKDTVLPRRAIEREEGQLQLCSRHRES
|
|
|
|---|
| BDBM50069490 |
|---|
| n/a |
|---|
| Name | BDBM50069490 |
|---|
| Synonyms: | CHEMBL147855 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrahydro-quinoxalin-5-ylmethyl)-2-(3-nitro-phenyl)-acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H13BrN4O5 |
|---|
| Mol. Mass. | 433.213 |
|---|
| SMILES | [O-][N+](=O)c1cccc(CC(=O)NCc2cc(Br)cc3[nH]c(=O)c(=O)[nH]c23)c1 |
|---|
| Structure | 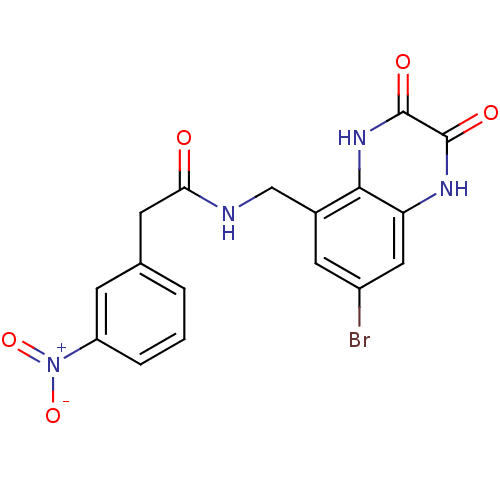
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Acklin, P; Allgeier, H; Auberson, YP; Bischoff, S; Ofner, S; Sauer, D; Schmutz, M 5-Aminomethylquinoxaline-2,3-diones, Part III: Arylamide derivatives as highly potent and selective glycine-site NMDA receptor antagonists. Bioorg Med Chem Lett8:493-8 (1999) [PubMed]
Acklin, P; Allgeier, H; Auberson, YP; Bischoff, S; Ofner, S; Sauer, D; Schmutz, M 5-Aminomethylquinoxaline-2,3-diones, Part III: Arylamide derivatives as highly potent and selective glycine-site NMDA receptor antagonists. Bioorg Med Chem Lett8:493-8 (1999) [PubMed]