| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Muscarinic acetylcholine receptor M2 |
|---|
| Ligand | BDBM50004734 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_98895 (CHEMBL703403) |
|---|
| Ki | 180±n/a nM |
|---|
| Citation |  Trybulski, EJ; Zhang, J; Kramss, RH; Mangano, RM The synthesis and biochemical pharmacology of enantiomerically pure methylated oxotremorine derivatives. J Med Chem36:3533-41 (1994) [PubMed] Trybulski, EJ; Zhang, J; Kramss, RH; Mangano, RM The synthesis and biochemical pharmacology of enantiomerically pure methylated oxotremorine derivatives. J Med Chem36:3533-41 (1994) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Muscarinic acetylcholine receptor M2 |
|---|
| Name: | Muscarinic acetylcholine receptor M2 |
|---|
| Synonyms: | ACM2_RAT | Cholinergic, muscarinic M2 | Chrm-2 | Chrm2 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 51555.53 |
|---|
| Organism: | RAT |
|---|
| Description: | P10980 |
|---|
| Residue: | 466 |
|---|
| Sequence: | MNNSTNSSNNGLAITSPYKTFEVVFIVLVAGSLSLVTIIGNILVMVSIKVNRHLQTVNNY
FLFSLACADLIIGVFSMNLYTLYTVIGYWPLGPVVCDLWLALDYVVSNASVMNLLIISFD
RYFCVTKPLTYPVKRTTKMAGMMIAAAWVLSFILWAPAILFWQFIVGVRTVEDGECYIQF
FSNAAVTFGTAIAAFYLPVIIMTVLYWHISRASKSRIKKEKKEPVANQDPVSPSLVQGRI
VKPNNNNMPGGDGGLEHNKIQNGKAPRDGVTENCVQGEEKESSNDSTSVSAVASNMRDDE
ITQDENTVSTSLGHSRDDNSKQTCIKIVTKAQKGDVCTPTSTTVELVGSSGQNGDEKQNI
VARKIVKMTKQPAKKKPPPSREKKVTRTILAILLAFIITWAPYNVMVLINTFCAPCIPNT
VWTIGYWLCYINSTINPACYALCNATFKKTFKHLLMCHYKNIGATR
|
|
|
|---|
| BDBM50004734 |
|---|
| n/a |
|---|
| Name | BDBM50004734 |
|---|
| Synonyms: | CHEMBL310852 | N-Methyl-N-(1-methyl-4-pyrrolidin-1-yl-but-2-ynyl)-acetamide | N-Methyl-N-(1-methyl-4-pyrrolidin-1-yl-but-2-ynyl)-acetamide (BM 5) | N-Methyl-N-(1-methyl-4-pyrrolidin-1-yl-but-2-ynyl)-acetamide (R) | N-Methyl-N-(1-methyl-4-pyrrolidin-1-yl-but-2-ynyl)-acetamide (S) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H20N2O |
|---|
| Mol. Mass. | 208.3 |
|---|
| SMILES | CC(C#CCN1CCCC1)N(C)C(C)=O |
|---|
| Structure | 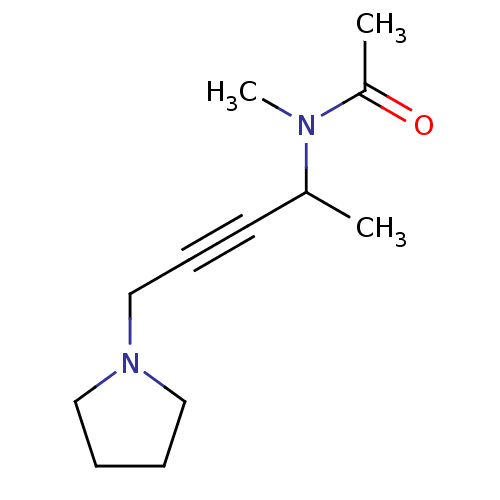
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Trybulski, EJ; Zhang, J; Kramss, RH; Mangano, RM The synthesis and biochemical pharmacology of enantiomerically pure methylated oxotremorine derivatives. J Med Chem36:3533-41 (1994) [PubMed]
Trybulski, EJ; Zhang, J; Kramss, RH; Mangano, RM The synthesis and biochemical pharmacology of enantiomerically pure methylated oxotremorine derivatives. J Med Chem36:3533-41 (1994) [PubMed]