| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1B |
|---|
| Ligand | BDBM50122816 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1336 (CHEMBL616962) |
|---|
| Ki | 2.8±n/a nM |
|---|
| Citation |  Balle, T; Perregaard, J; Ramirez, MT; Larsen, AK; Søby, KK; Liljefors, T; Andersen, K Synthesis and structure-affinity relationship investigations of 5-heteroaryl-substituted analogues of the antipsychotic sertindole. A new class of highly selective alpha(1) adrenoceptor antagonists. J Med Chem46:265-83 (2003) [PubMed] Article Balle, T; Perregaard, J; Ramirez, MT; Larsen, AK; Søby, KK; Liljefors, T; Andersen, K Synthesis and structure-affinity relationship investigations of 5-heteroaryl-substituted analogues of the antipsychotic sertindole. A new class of highly selective alpha(1) adrenoceptor antagonists. J Med Chem46:265-83 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1B |
|---|
| Name: | 5-hydroxytryptamine receptor 1B |
|---|
| Synonyms: | 5-HT-1B | 5-HT-1D-beta | 5-HT1B | 5-hydroxytryptamine receptor 1B (5-HT1B) | 5HT1B_HUMAN | HTR1B | HTR1DB | S12 | Serotonin (5-HT) receptor | Serotonin 1D beta receptor | Serotonin Receptor 1B |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 43579.17 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Receptor binding assays were performed using human clone stably expressed in CHO cells |
|---|
| Residue: | 390 |
|---|
| Sequence: | MEEPGAQCAPPPPAGSETWVPQANLSSAPSQNCSAKDYIYQDSISLPWKVLLVMLLALIT
LATTLSNAFVIATVYRTRKLHTPANYLIASLAVTDLLVSILVMPISTMYTVTGRWTLGQV
VCDFWLSSDITCCTASILHLCVIALDRYWAITDAVEYSAKRTPKRAAVMIALVWVFSISI
SLPPFFWRQAKAEEEVSECVVNTDHILYTVYSTVGAFYFPTLLLIALYGRIYVEARSRIL
KQTPNRTGKRLTRAQLITDSPGSTSSVTSINSRVPDVPSESGSPVYVNQVKVRVSDALLE
KKKLMAARERKATKTLGIILGAFIVCWLPFFIISLVMPICKDACWFHLAIFDFFTWLGYL
NSLINPIIYTMSNEDFKQAFHKLIRFKCTS
|
|
|
|---|
| BDBM50122816 |
|---|
| n/a |
|---|
| Name | BDBM50122816 |
|---|
| Synonyms: | 1-(2-{4-[1-(4-Fluoro-phenyl)-5-(3-methyl-3H-[1,2,3]triazol-4-yl)-1H-indol-3-yl]-piperidin-1-yl}-ethyl)-imidazolidin-2-one | CHEMBL97184 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H30FN7O |
|---|
| Mol. Mass. | 487.5718 |
|---|
| SMILES | Cn1nncc1-c1ccc2n(cc(C3CCN(CCN4CCNC4=O)CC3)c2c1)-c1ccc(F)cc1 |
|---|
| Structure | 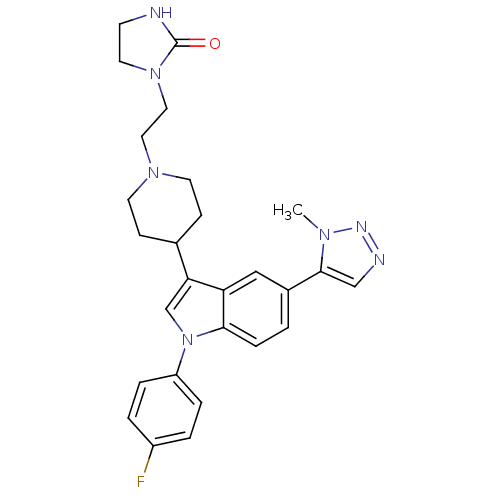
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Balle, T; Perregaard, J; Ramirez, MT; Larsen, AK; Søby, KK; Liljefors, T; Andersen, K Synthesis and structure-affinity relationship investigations of 5-heteroaryl-substituted analogues of the antipsychotic sertindole. A new class of highly selective alpha(1) adrenoceptor antagonists. J Med Chem46:265-83 (2003) [PubMed] Article
Balle, T; Perregaard, J; Ramirez, MT; Larsen, AK; Søby, KK; Liljefors, T; Andersen, K Synthesis and structure-affinity relationship investigations of 5-heteroaryl-substituted analogues of the antipsychotic sertindole. A new class of highly selective alpha(1) adrenoceptor antagonists. J Med Chem46:265-83 (2003) [PubMed] Article