| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50332991 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_476993 (CHEMBL937498) |
|---|
| IC50 | 4300±n/a nM |
|---|
| Citation |  Woltering, TJ; Wichmann, J; Goetschi, E; Adam, G; Kew, JN; Knoflach, F; Ballard, TM; Huwyler, J; Mutel, V; Gatti, S Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg Med Chem Lett18:2725-9 (2008) [PubMed] Article Woltering, TJ; Wichmann, J; Goetschi, E; Adam, G; Kew, JN; Knoflach, F; Ballard, TM; Huwyler, J; Mutel, V; Gatti, S Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg Med Chem Lett18:2725-9 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50332991 |
|---|
| n/a |
|---|
| Name | BDBM50332991 |
|---|
| Synonyms: | 4-(3-(1H-1,2,3-triazol-1-yl)phenyl)-8-(2-fluorophenyl)-1H-benzo[b][1,4]diazepin-2(3H)-one | CHEMBL261288 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H16FN5O |
|---|
| Mol. Mass. | 397.4044 |
|---|
| SMILES | Fc1ccccc1-c1ccc2N=C(CC(=O)Nc2c1)c1cccc(c1)-n1ccnn1 |c:12| |
|---|
| Structure | 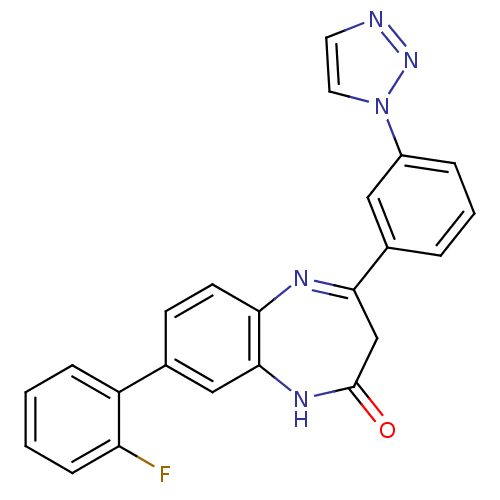
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Woltering, TJ; Wichmann, J; Goetschi, E; Adam, G; Kew, JN; Knoflach, F; Ballard, TM; Huwyler, J; Mutel, V; Gatti, S Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg Med Chem Lett18:2725-9 (2008) [PubMed] Article
Woltering, TJ; Wichmann, J; Goetschi, E; Adam, G; Kew, JN; Knoflach, F; Ballard, TM; Huwyler, J; Mutel, V; Gatti, S Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg Med Chem Lett18:2725-9 (2008) [PubMed] Article