| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Mitogen-activated protein kinase 8 |
|---|
| Ligand | BDBM50303636 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_596582 (CHEMBL1042601) |
|---|
| IC50 | 535±n/a nM |
|---|
| Citation |  Kamenecka, T; Jiang, R; Song, X; Duckett, D; Chen, W; Ling, YY; Habel, J; Laughlin, JD; Chambers, J; Figuera-Losada, M; Cameron, MD; Lin, L; Ruiz, CH; LoGrasso, PV Synthesis, biological evaluation, X-ray structure, and pharmacokinetics of aminopyrimidine c-jun-N-terminal kinase (JNK) inhibitors. J Med Chem53:419-31 (2010) [PubMed] Article Kamenecka, T; Jiang, R; Song, X; Duckett, D; Chen, W; Ling, YY; Habel, J; Laughlin, JD; Chambers, J; Figuera-Losada, M; Cameron, MD; Lin, L; Ruiz, CH; LoGrasso, PV Synthesis, biological evaluation, X-ray structure, and pharmacokinetics of aminopyrimidine c-jun-N-terminal kinase (JNK) inhibitors. J Med Chem53:419-31 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Mitogen-activated protein kinase 8 |
|---|
| Name: | Mitogen-activated protein kinase 8 |
|---|
| Synonyms: | JNK-46 | JNK1 | JNK1-alpha-1 | MAPK8 | MK08_HUMAN | Mitogen-Activated Protein Kinase 8 (JNK1) | PRKM8 | SAPK1 | SAPK1C | Stress-activated protein kinase JNK1 | c-Jun N-terminal kinase 1 | c-Jun N-terminal kinase 1 (JNK1) | c-Jun N-terminal kinase 1(JNK1) | c-Jun N-terminal kinase 2 (JNK2) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48297.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | JNK-1 was purchased from Upstate Cell Signaling Solutions (formerly Upstate Biotechnology). |
|---|
| Residue: | 427 |
|---|
| Sequence: | MSRSKRDNNFYSVEIGDSTFTVLKRYQNLKPIGSGAQGIVCAAYDAILERNVAIKKLSRP
FQNQTHAKRAYRELVLMKCVNHKNIIGLLNVFTPQKSLEEFQDVYIVMELMDANLCQVIQ
MELDHERMSYLLYQMLCGIKHLHSAGIIHRDLKPSNIVVKSDCTLKILDFGLARTAGTSF
MMTPYVVTRYYRAPEVILGMGYKENVDLWSVGCIMGEMVCHKILFPGRDYIDQWNKVIEQ
LGTPCPEFMKKLQPTVRTYVENRPKYAGYSFEKLFPDVLFPADSEHNKLKASQARDLLSK
MLVIDASKRISVDEALQHPYINVWYDPSEAEAPPPKIPDKQLDEREHTIEEWKELIYKEV
MDLEERTKNGVIRGQPSPLGAAVINGSQHPSSSSSVNDVSSMSTDPTLASDTDSSLEAAA
GPLGCCR
|
|
|
|---|
| BDBM50303636 |
|---|
| n/a |
|---|
| Name | BDBM50303636 |
|---|
| Synonyms: | 4-(4-Phenylpyrimidin-2-ylamino)benzamide | CHEMBL571619 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H14N4O |
|---|
| Mol. Mass. | 290.3193 |
|---|
| SMILES | NC(=O)c1ccc(Nc2nccc(n2)-c2ccccc2)cc1 |
|---|
| Structure | 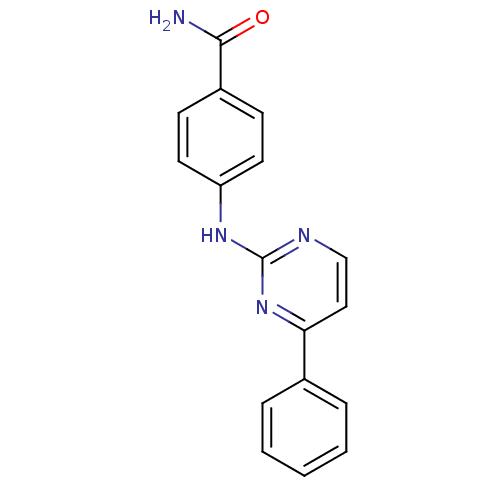
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kamenecka, T; Jiang, R; Song, X; Duckett, D; Chen, W; Ling, YY; Habel, J; Laughlin, JD; Chambers, J; Figuera-Losada, M; Cameron, MD; Lin, L; Ruiz, CH; LoGrasso, PV Synthesis, biological evaluation, X-ray structure, and pharmacokinetics of aminopyrimidine c-jun-N-terminal kinase (JNK) inhibitors. J Med Chem53:419-31 (2010) [PubMed] Article
Kamenecka, T; Jiang, R; Song, X; Duckett, D; Chen, W; Ling, YY; Habel, J; Laughlin, JD; Chambers, J; Figuera-Losada, M; Cameron, MD; Lin, L; Ruiz, CH; LoGrasso, PV Synthesis, biological evaluation, X-ray structure, and pharmacokinetics of aminopyrimidine c-jun-N-terminal kinase (JNK) inhibitors. J Med Chem53:419-31 (2010) [PubMed] Article