 Found 817 hits with Last Name = 'duckett' and Initial = 'd'
Found 817 hits with Last Name = 'duckett' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577925

(CHEMBL4846565)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1cn[nH]c1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577932
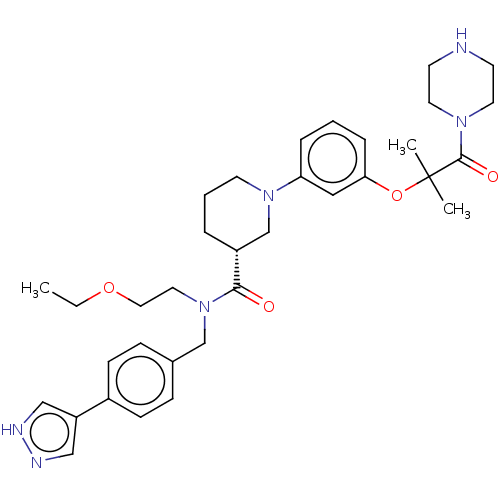
(CHEMBL4853982)Show SMILES Cl.CCOCCN(Cc1ccc(cc1)-c1cn[nH]c1)C(=O)[C@@H]1CCCN(C1)c1cccc(OC(C)(C)C(=O)N2CCNCC2)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577931

(CHEMBL4863751)Show SMILES Cl.CCN(Cc1ccc(cc1)-c1cn[nH]c1)C(=O)[C@@H]1CCCN(C1)c1cccc(OC(C)(C)C(=O)N2CCNCC2)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577917

(CHEMBL4874860)Show SMILES Cl.CC(C)c1ccc(CN(C2CC2)C(=O)[C@@H]2CCCN(C2)c2cccc(OC(C)(C)C(=O)N3CCNCC3)c2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577920
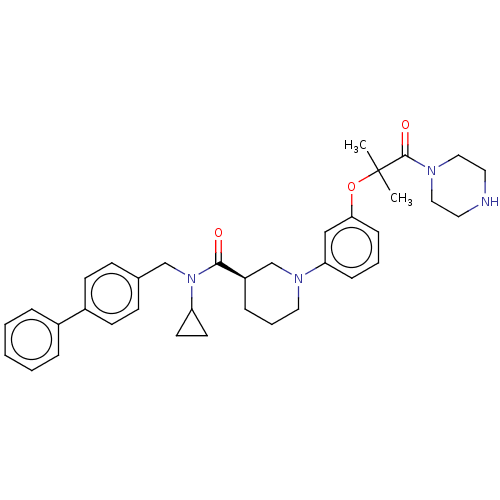
(CHEMBL4857780)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1ccccc1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577916
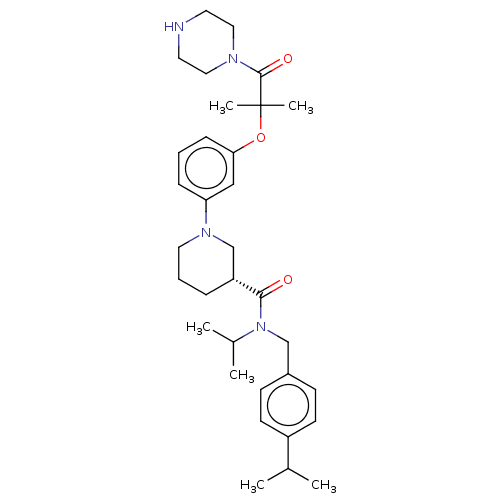
(CHEMBL4851887)Show SMILES Cl.CC(C)N(Cc1ccc(cc1)C(C)C)C(=O)[C@@H]1CCCN(C1)c1cccc(OC(C)(C)C(=O)N2CCNCC2)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577919

(CHEMBL4869003)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)C1CCCCC1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577921

(CHEMBL4860362)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1ccsc1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577912

(CHEMBL4852661)Show SMILES Cl.CC(C)c1ccc(CNC(=O)[C@@H]2CCCN(C2)c2cccc(OC(C)(C)C(=O)N3CCNCC3)c2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577927

(CHEMBL4876911)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1ccn[nH]1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577925

(CHEMBL4846565)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1cn[nH]c1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin R1C domain (138 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577915

(CHEMBL4852707)Show SMILES Cl.CCN(Cc1ccc(cc1)C(C)C)C(=O)[C@@H]1CCCN(C1)c1cccc(OC(C)(C)C(=O)N2CCNCC2)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577918
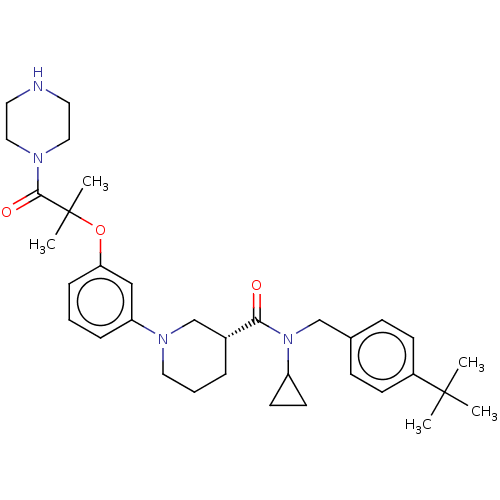
(CHEMBL4869538)Show SMILES Cl.CC(C)(C)c1ccc(CN(C2CC2)C(=O)[C@@H]2CCCN(C2)c2cccc(OC(C)(C)C(=O)N3CCNCC3)c2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577911

(CHEMBL4862286)Show SMILES Cl.CC(C)c1ccc(CN(C)C(=O)[C@@H]2CCCN(C2)c2cccc(OC(C)(C)C(=O)N3CCNCC3)c2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577929
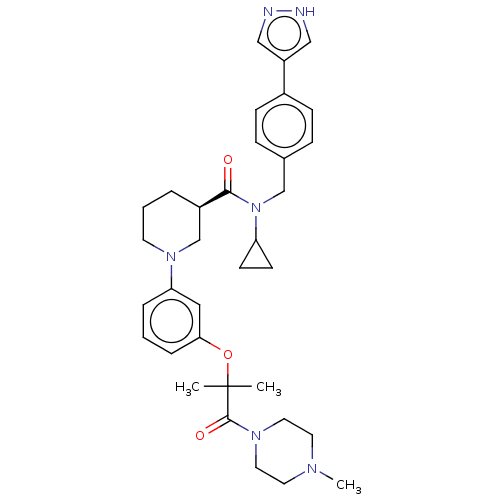
(CHEMBL4847343)Show SMILES CN1CCN(CC1)C(=O)C(C)(C)Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1cn[nH]c1)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577926

(CHEMBL4871689)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@@H](C1)C(=O)N(Cc1ccc(cc1)-c1cn[nH]c1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577930
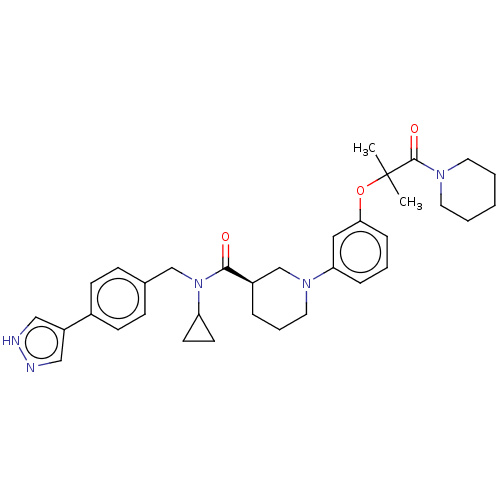
(CHEMBL4855433)Show SMILES CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1cn[nH]c1)C1CC1)C(=O)N1CCCCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577913

(CHEMBL4873635)Show SMILES CC(C)c1ccc(CNC(=O)[C@@H]2CCCN(C2)c2cccc(OC(C)(C)C(O)=O)c2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577922
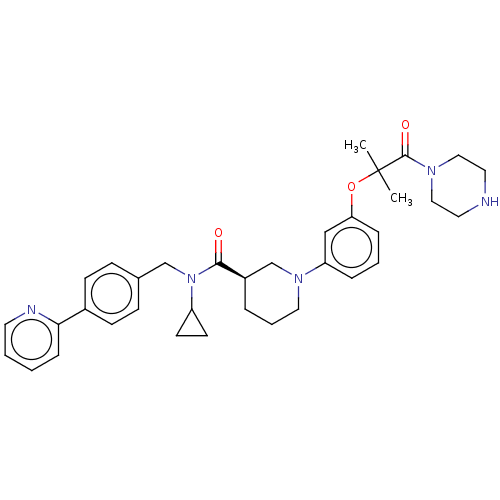
(CHEMBL4869034)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1ccccn1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577923

(CHEMBL4868531)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1cccnc1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577928
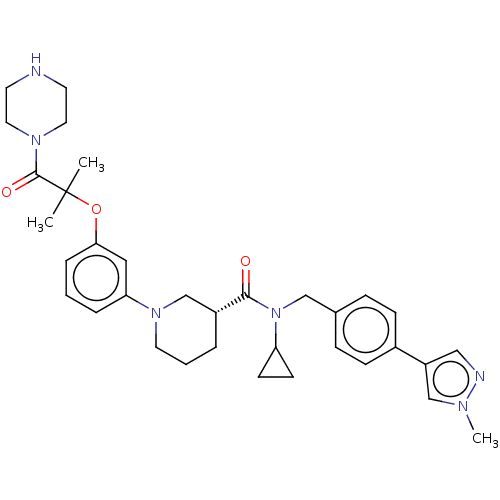
(CHEMBL4864467)Show SMILES Cl.Cn1cc(cn1)-c1ccc(CN(C2CC2)C(=O)[C@@H]2CCCN(C2)c2cccc(OC(C)(C)C(=O)N3CCNCC3)c2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577924

(CHEMBL4858087)Show SMILES Cl.CC(C)(Oc1cccc(c1)N1CCC[C@H](C1)C(=O)N(Cc1ccc(cc1)-c1ccncc1)C1CC1)C(=O)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
B-cell CLL/lymphoma 9 protein
(Homo sapiens) | BDBM50577914

(CHEMBL4868665)Show SMILES CC(C)c1ccc(CNC(=O)[C@H]2CCCN(C2)c2cccc(OC(C)(C)C(O)=O)c2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-terminal biotinyla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00742
BindingDB Entry DOI: 10.7270/Q2SN0DSK |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50311713
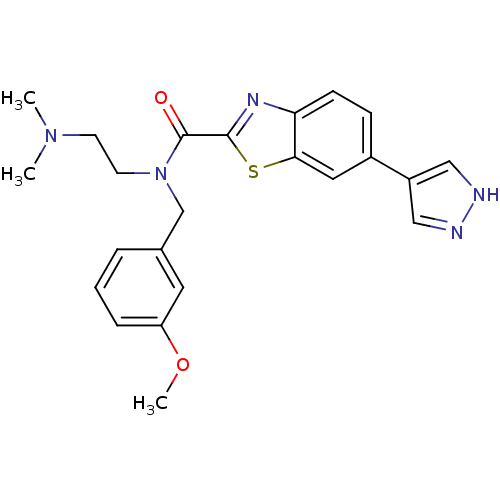
(CHEMBL1080279 | N-(2-(dimethylamino)ethyl)-N-(3-me...)Show SMILES COc1cccc(CN(CCN(C)C)C(=O)c2nc3ccc(cc3s2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C23H25N5O2S/c1-27(2)9-10-28(15-16-5-4-6-19(11-16)30-3)23(29)22-26-20-8-7-17(12-21(20)31-22)18-13-24-25-14-18/h4-8,11-14H,9-10,15H2,1-3H3,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 19: 6686-90 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.115
BindingDB Entry DOI: 10.7270/Q2VX0GN0 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50311728
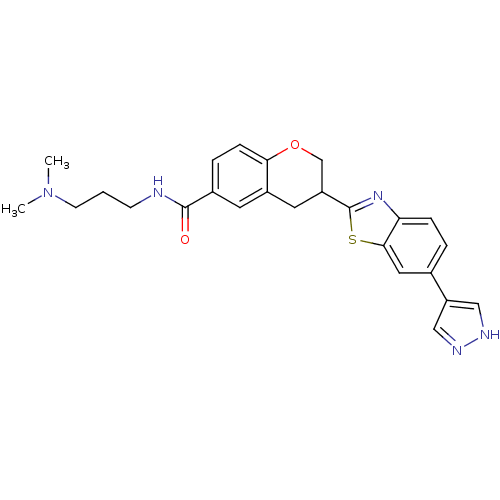
(3-(6-(1H-pyrazol-4-yl)benzo[d]thiazol-2-yl)-N-(3-(...)Show SMILES CN(C)CCCNC(=O)c1ccc2OCC(Cc2c1)c1nc2ccc(cc2s1)-c1cn[nH]c1 Show InChI InChI=1S/C25H27N5O2S/c1-30(2)9-3-8-26-24(31)17-5-7-22-18(10-17)11-19(15-32-22)25-29-21-6-4-16(12-23(21)33-25)20-13-27-28-14-20/h4-7,10,12-14,19H,3,8-9,11,15H2,1-2H3,(H,26,31)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 19: 6686-90 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.115
BindingDB Entry DOI: 10.7270/Q2VX0GN0 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM25004
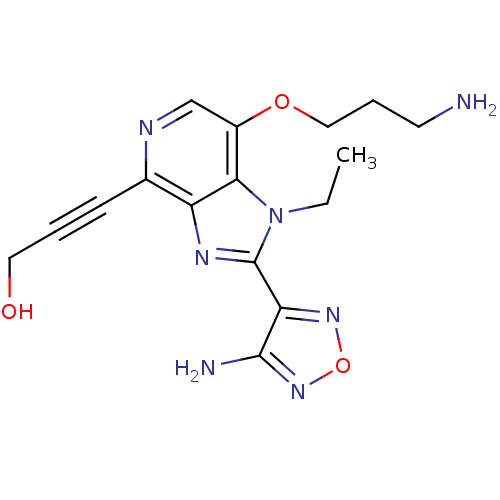
(3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...)Show InChI InChI=1S/C16H19N7O3/c1-2-23-14-11(25-8-4-6-17)9-19-10(5-3-7-24)12(14)20-16(23)13-15(18)22-26-21-13/h9,24H,2,4,6-8,17H2,1H3,(H2,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24994
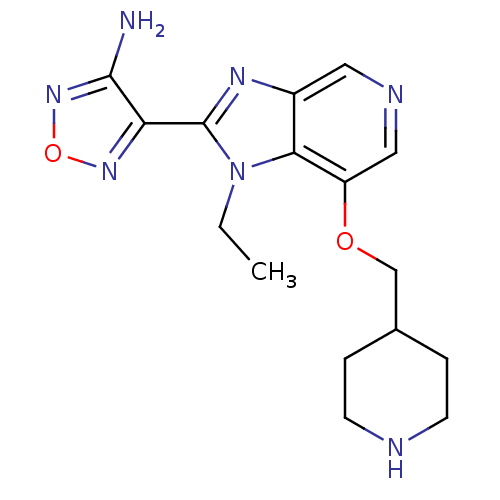
(4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...)Show InChI InChI=1S/C16H21N7O2/c1-2-23-14-11(20-16(23)13-15(17)22-25-21-13)7-19-8-12(14)24-9-10-3-5-18-6-4-10/h7-8,10,18H,2-6,9H2,1H3,(H2,17,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50495031

(CHEMBL3099975)Show SMILES Oc1onc(c1-c1ccc(F)cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)c1 Show InChI InChI=1S/C24H21FN4O3/c25-18-3-1-16(2-4-18)22-23(28-32-24(22)30)17-9-10-26-21(15-17)27-19-5-7-20(8-6-19)29-11-13-31-14-12-29/h1-10,15,30H,11-14H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 (39 to 402 amino acids) (unknown origin) expressed in Escherichia coli BL21(DE3) using biotinylated ATF2 as substrate after 15 min... |
Bioorg Med Chem Lett 24: 161-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.052
BindingDB Entry DOI: 10.7270/Q2XG9V3X |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256436

(CHEMBL481735 | N-(2-(2-(dimethylamino)ethoxy)-4-(1...)Show SMILES CN(C)CCOc1cc(ccc1NC(=O)C1COc2ccc(C)cc2C1)-c1cn[nH]c1 Show InChI InChI=1S/C24H28N4O3/c1-16-4-7-22-18(10-16)11-19(15-31-22)24(29)27-21-6-5-17(20-13-25-26-14-20)12-23(21)30-9-8-28(2)3/h4-7,10,12-14,19H,8-9,11,15H2,1-3H3,(H,25,26)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
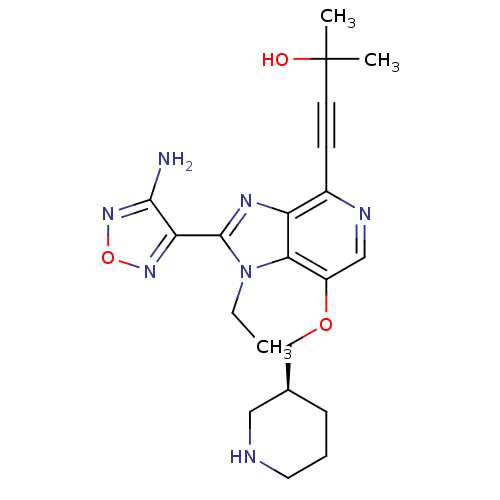
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50311728

(3-(6-(1H-pyrazol-4-yl)benzo[d]thiazol-2-yl)-N-(3-(...)Show SMILES CN(C)CCCNC(=O)c1ccc2OCC(Cc2c1)c1nc2ccc(cc2s1)-c1cn[nH]c1 Show InChI InChI=1S/C25H27N5O2S/c1-30(2)9-3-8-26-24(31)17-5-7-22-18(10-17)11-19(15-32-22)25-29-21-6-4-16(12-23(21)33-25)20-13-27-28-14-20/h4-7,10,12-14,19H,3,8-9,11,15H2,1-2H3,(H,26,31)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 |
Bioorg Med Chem Lett 19: 6686-90 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.115
BindingDB Entry DOI: 10.7270/Q2VX0GN0 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256384
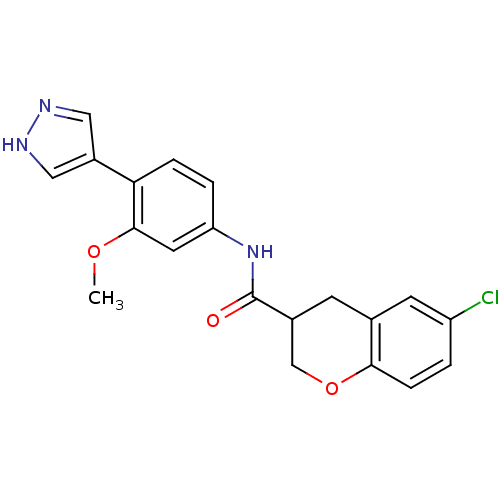
(6-chloro-N-(3-methoxy-4-(1H-pyrazol-4-yl)phenyl)ch...)Show SMILES COc1cc(NC(=O)C2COc3ccc(Cl)cc3C2)ccc1-c1cn[nH]c1 Show InChI InChI=1S/C20H18ClN3O3/c1-26-19-8-16(3-4-17(19)14-9-22-23-10-14)24-20(25)13-6-12-7-15(21)2-5-18(12)27-11-13/h2-5,7-10,13H,6,11H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256322
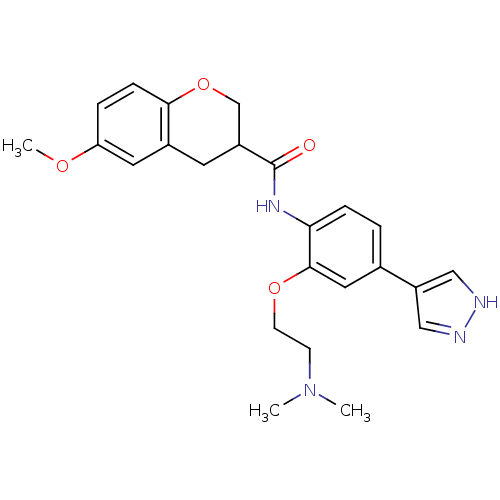
(CHEMBL482336 | N-(2-(2-(dimethylamino)ethoxy)-4-(1...)Show SMILES COc1ccc2OCC(Cc2c1)C(=O)Nc1ccc(cc1OCCN(C)C)-c1cn[nH]c1 Show InChI InChI=1S/C24H28N4O4/c1-28(2)8-9-31-23-12-16(19-13-25-26-14-19)4-6-21(23)27-24(29)18-10-17-11-20(30-3)5-7-22(17)32-15-18/h4-7,11-14,18H,8-10,15H2,1-3H3,(H,25,26)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25009
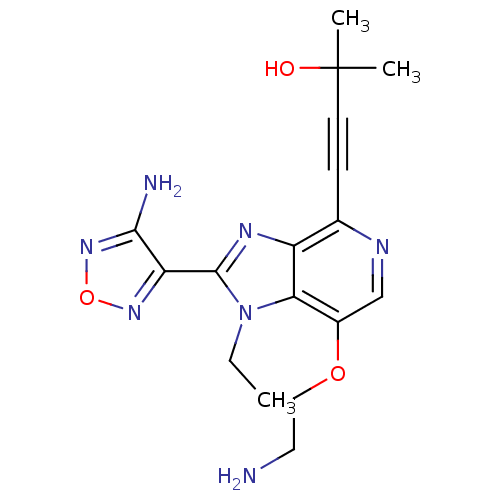
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(2-aminoetho...)Show SMILES CCn1c(nc2c(ncc(OCCN)c12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-14-11(26-8-7-18)9-20-10(5-6-17(2,3)25)12(14)21-16(24)13-15(19)23-27-22-13/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25004

(3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...)Show InChI InChI=1S/C16H19N7O3/c1-2-23-14-11(25-8-4-6-17)9-19-10(5-3-7-24)12(14)20-16(23)13-15(18)22-26-21-13/h9,24H,2,4,6-8,17H2,1H3,(H2,18,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256383
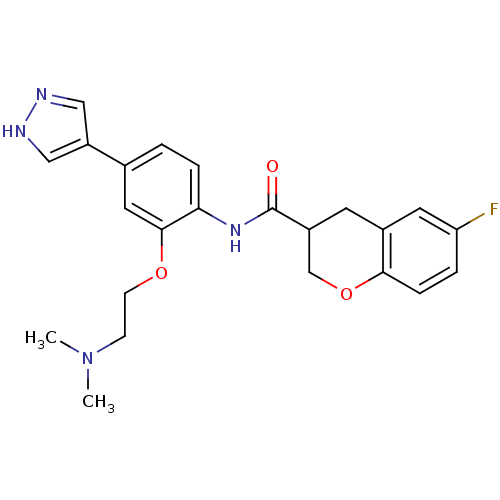
(CHEMBL481545 | N-(2-(2-(dimethylamino)ethoxy)-4-(1...)Show SMILES CN(C)CCOc1cc(ccc1NC(=O)C1COc2ccc(F)cc2C1)-c1cn[nH]c1 Show InChI InChI=1S/C23H25FN4O3/c1-28(2)7-8-30-22-11-15(18-12-25-26-13-18)3-5-20(22)27-23(29)17-9-16-10-19(24)4-6-21(16)31-14-17/h3-6,10-13,17H,7-9,14H2,1-2H3,(H,25,26)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256067
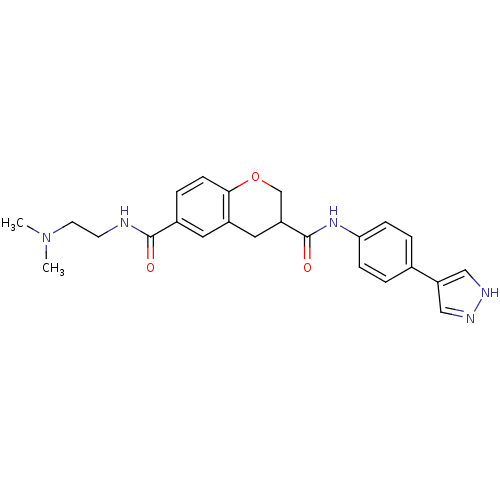
(CHEMBL481685 | N3-(4-(1H-pyrazol-4-yl)phenyl)-N6-(...)Show SMILES CN(C)CCNC(=O)c1ccc2OCC(Cc2c1)C(=O)Nc1ccc(cc1)-c1cn[nH]c1 Show InChI InChI=1S/C24H27N5O3/c1-29(2)10-9-25-23(30)17-5-8-22-18(11-17)12-19(15-32-22)24(31)28-21-6-3-16(4-7-21)20-13-26-27-14-20/h3-8,11,13-14,19H,9-10,12,15H2,1-2H3,(H,25,30)(H,26,27)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM25472
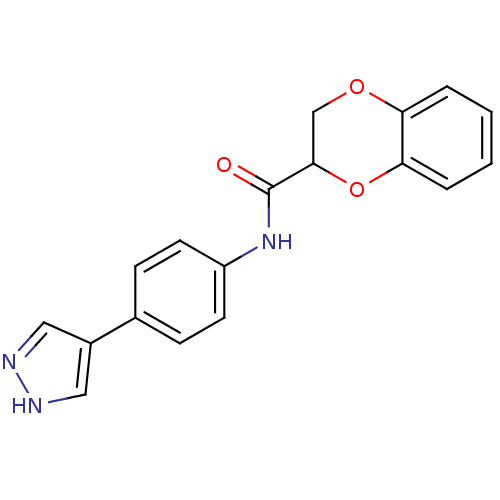
(CHEMBL519123 | N-[4-(1H-pyrazol-4-yl)phenyl]-2,3-d...)Show InChI InChI=1S/C18H15N3O3/c22-18(17-11-23-15-3-1-2-4-16(15)24-17)21-14-7-5-12(6-8-14)13-9-19-20-10-13/h1-10,17H,11H2,(H,19,20)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50246409

(CHEMBL455658 | N-(4-(1H-pyrazol-4-yl)phenyl)-3,4-d...)Show InChI InChI=1S/C19H17N3O2/c23-19(15-9-14-3-1-2-4-18(14)24-12-15)22-17-7-5-13(6-8-17)16-10-20-21-11-16/h1-8,10-11,15H,9,12H2,(H,20,21)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256111
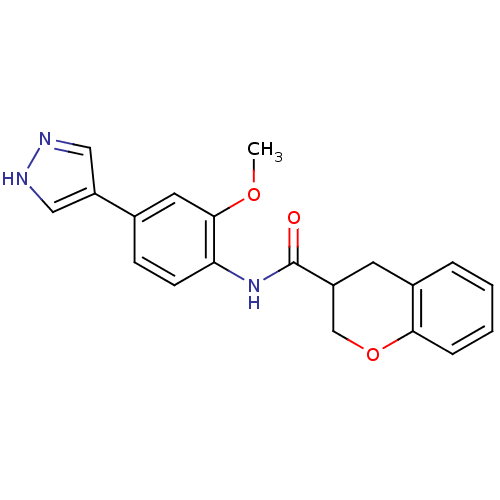
(CHEMBL480586 | N-(2-methoxy-4-(1H-pyrazol-4-yl)phe...)Show SMILES COc1cc(ccc1NC(=O)C1COc2ccccc2C1)-c1cn[nH]c1 Show InChI InChI=1S/C20H19N3O3/c1-25-19-9-13(16-10-21-22-11-16)6-7-17(19)23-20(24)15-8-14-4-2-3-5-18(14)26-12-15/h2-7,9-11,15H,8,12H2,1H3,(H,21,22)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256068

(6-methoxy-N-(3-methoxy-4-(1H-pyrazol-4-yl)phenyl)c...)Show SMILES COc1ccc2OCC(Cc2c1)C(=O)Nc1ccc(-c2cn[nH]c2)c(OC)c1 Show InChI InChI=1S/C21H21N3O4/c1-26-17-4-6-19-13(8-17)7-14(12-28-19)21(25)24-16-3-5-18(20(9-16)27-2)15-10-22-23-11-15/h3-6,8-11,14H,7,12H2,1-2H3,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256385
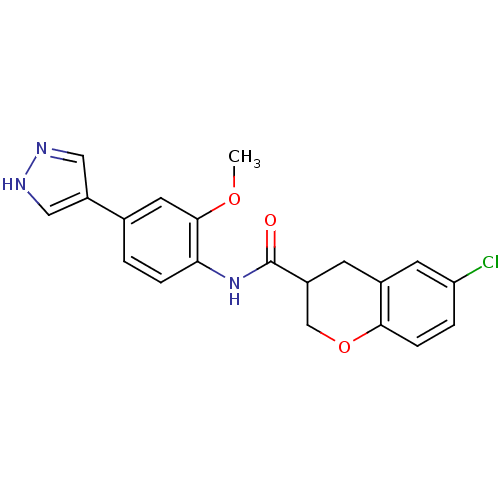
(6-chloro-N-(2-methoxy-4-(1H-pyrazol-4-yl)phenyl)ch...)Show SMILES COc1cc(ccc1NC(=O)C1COc2ccc(Cl)cc2C1)-c1cn[nH]c1 Show InChI InChI=1S/C20H18ClN3O3/c1-26-19-8-12(15-9-22-23-10-15)2-4-17(19)24-20(25)14-6-13-7-16(21)3-5-18(13)27-11-14/h2-5,7-10,14H,6,11H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256163
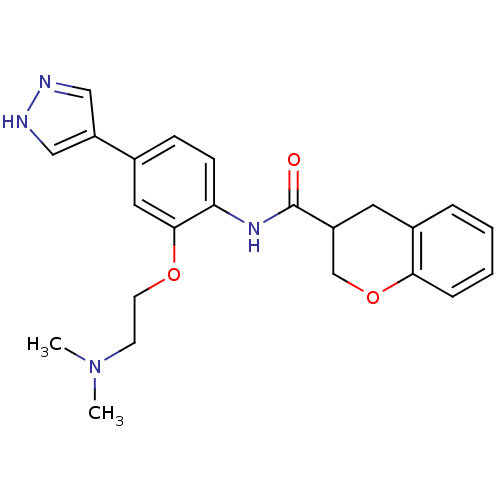
(CHEMBL474425 | N-(2-(2-(dimethylamino)ethoxy)-4-(1...)Show SMILES CN(C)CCOc1cc(ccc1NC(=O)C1COc2ccccc2C1)-c1cn[nH]c1 Show InChI InChI=1S/C23H26N4O3/c1-27(2)9-10-29-22-12-16(19-13-24-25-14-19)7-8-20(22)26-23(28)18-11-17-5-3-4-6-21(17)30-15-18/h3-8,12-14,18H,9-11,15H2,1-2H3,(H,24,25)(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM24994

(4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...)Show InChI InChI=1S/C16H21N7O2/c1-2-23-14-11(20-16(23)13-15(17)22-25-21-13)7-19-8-12(14)24-9-10-3-5-18-6-4-10/h7-8,10,18H,2-6,9H2,1H3,(H2,17,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24991
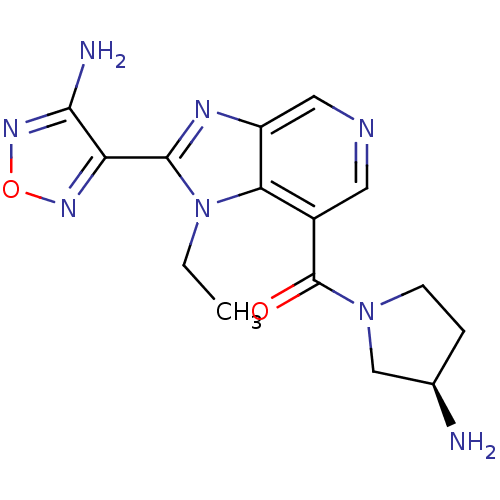
(4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...)Show SMILES CCn1c(nc2cncc(C(=O)N3CC[C@@H](N)C3)c12)-c1nonc1N |r| Show InChI InChI=1S/C15H18N8O2/c1-2-23-12-9(15(24)22-4-3-8(16)7-22)5-18-6-10(12)19-14(23)11-13(17)21-25-20-11/h5-6,8H,2-4,7,16H2,1H3,(H2,17,21)/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25016
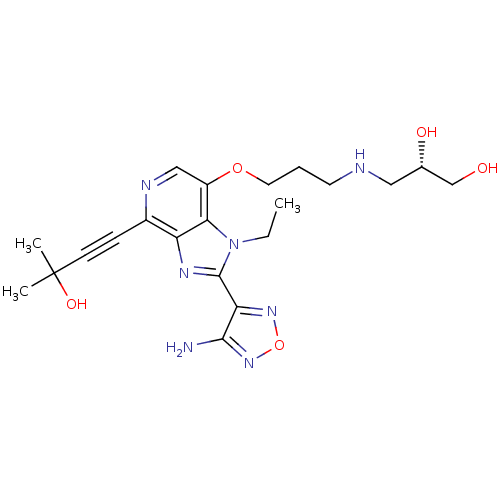
((2S)-3-[(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-et...)Show SMILES CCn1c(nc2c(ncc(OCCCNC[C@H](O)CO)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H29N7O5/c1-4-28-18-15(32-9-5-8-23-10-13(30)12-29)11-24-14(6-7-21(2,3)31)16(18)25-20(28)17-19(22)27-33-26-17/h11,13,23,29-31H,4-5,8-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25014
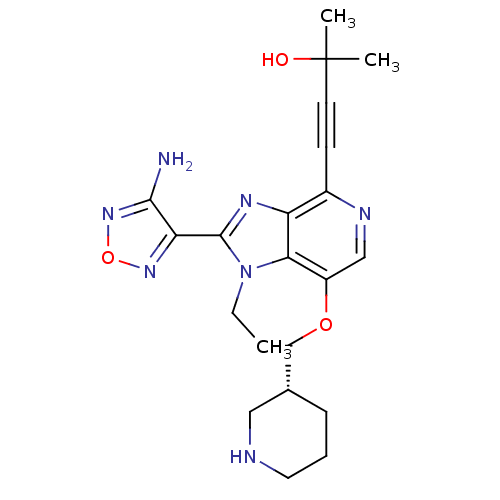
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25010

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-[(3S)-3-amin...)Show SMILES CCn1c(nc2c(ncc(OCC[C@@H](N)Cc3ccccc3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C25H29N7O3/c1-4-32-22-19(34-13-11-17(26)14-16-8-6-5-7-9-16)15-28-18(10-12-25(2,3)33)20(22)29-24(32)21-23(27)31-35-30-21/h5-9,15,17,33H,4,11,13-14,26H2,1-3H3,(H2,27,31)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256166
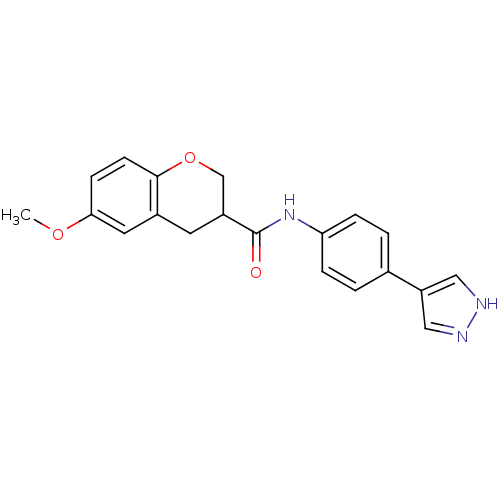
(CHEMBL475601 | N-(4-(1H-pyrazol-4-yl)phenyl)-6-met...)Show SMILES COc1ccc2OCC(Cc2c1)C(=O)Nc1ccc(cc1)-c1cn[nH]c1 Show InChI InChI=1S/C20H19N3O3/c1-25-18-6-7-19-14(9-18)8-15(12-26-19)20(24)23-17-4-2-13(3-5-17)16-10-21-22-11-16/h2-7,9-11,15H,8,12H2,1H3,(H,21,22)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50256382

(6-fluoro-N-(2-methoxy-4-(1H-pyrazol-4-yl)phenyl)ch...)Show SMILES COc1cc(ccc1NC(=O)C1COc2ccc(F)cc2C1)-c1cn[nH]c1 Show InChI InChI=1S/C20H18FN3O3/c1-26-19-8-12(15-9-22-23-10-15)2-4-17(19)24-20(25)14-6-13-7-16(21)3-5-18(13)27-11-14/h2-5,7-10,14H,6,11H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6406-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.080
BindingDB Entry DOI: 10.7270/Q2KK9BMC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data