| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Carbonic anhydrase 9 |
|---|
| Ligand | BDBM12920 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_625072 (CHEMBL1112521) |
|---|
| Ki | 1238±n/a nM |
|---|
| Citation |  Lopez, M; Bornaghi, LF; Innocenti, A; Vullo, D; Charman, SA; Supuran, CT; Poulsen, SA Sulfonamide linked neoglycoconjugates--a new class of inhibitors for cancer-associated carbonic anhydrases. J Med Chem53:2913-26 (2010) [PubMed] Article Lopez, M; Bornaghi, LF; Innocenti, A; Vullo, D; Charman, SA; Supuran, CT; Poulsen, SA Sulfonamide linked neoglycoconjugates--a new class of inhibitors for cancer-associated carbonic anhydrases. J Med Chem53:2913-26 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Carbonic anhydrase 9 |
|---|
| Name: | Carbonic anhydrase 9 |
|---|
| Synonyms: | CA-IX | CA9 | CAH9_HUMAN | Carbonate dehydratase IX | Carbonic anhydrase 9 (CA IX) | Carbonic anhydrase 9 (CAIX) | Carbonic anhydrase 9 precursor | Carbonic anhydrase IX (CA IX) | Carbonic anhydrase IX (CAIX) | Carbonic anhydrases IX | Carbonic anhydrases; II & IX | G250 | MN | Membrane antigen MN | RCC-associated antigen G250 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 49669.03 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Catalytic domain of human cloned isozyme was used in the assay |
|---|
| Residue: | 459 |
|---|
| Sequence: | MAPLCPSPWLPLLIPAPAPGLTVQLLLSLLLLVPVHPQRLPRMQEDSPLGGGSSGEDDPL
GEEDLPSEEDSPREEDPPGEEDLPGEEDLPGEEDLPEVKPKSEEEGSLKLEDLPTVEAPG
DPQEPQNNAHRDKEGDDQSHWRYGGDPPWPRVSPACAGRFQSPVDIRPQLAAFCPALRPL
ELLGFQLPPLPELRLRNNGHSVQLTLPPGLEMALGPGREYRALQLHLHWGAAGRPGSEHT
VEGHRFPAEIHVVHLSTAFARVDEALGRPGGLAVLAAFLEEGPEENSAYEQLLSRLEEIA
EEGSETQVPGLDISALLPSDFSRYFQYEGSLTTPPCAQGVIWTVFNQTVMLSAKQLHTLS
DTLWGPGDSRLQLNFRATQPLNGRVIEASFPAGVDSSPRAAEPVQLNSCLAAGDILALVF
GLLFAVTSVAFLVQMRRQHRRGTKGGVSYRPAEVAETGA
|
|
|
|---|
| BDBM12920 |
|---|
| n/a |
|---|
| Name | BDBM12920 |
|---|
| Synonyms: | 4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)-1H-1,2,3-triazole | CHEMBL383897 | Glycoconjugate Benzene Sulfonamide 3a | {1-[(2R,3R,4S,5R,6R)-3,4,5-tris(acetyloxy)-6-[(acetyloxy)methyl]oxan-2-yl]-1H-1,2,3-triazol-4-yl}methyl 4-sulfamoylbenzoate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H28N4O13S |
|---|
| Mol. Mass. | 612.563 |
|---|
| SMILES | CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(COC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 |
|---|
| Structure | 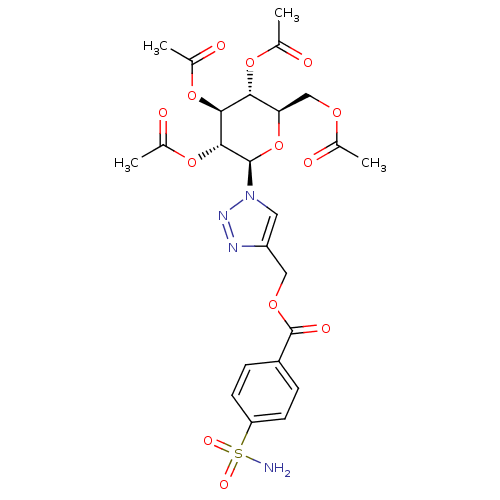
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lopez, M; Bornaghi, LF; Innocenti, A; Vullo, D; Charman, SA; Supuran, CT; Poulsen, SA Sulfonamide linked neoglycoconjugates--a new class of inhibitors for cancer-associated carbonic anhydrases. J Med Chem53:2913-26 (2010) [PubMed] Article
Lopez, M; Bornaghi, LF; Innocenti, A; Vullo, D; Charman, SA; Supuran, CT; Poulsen, SA Sulfonamide linked neoglycoconjugates--a new class of inhibitors for cancer-associated carbonic anhydrases. J Med Chem53:2913-26 (2010) [PubMed] Article