 Found 1591 hits with Last Name = 'lopez' and Initial = 'm'
Found 1591 hits with Last Name = 'lopez' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50083197

(6-Chloro-1H-benzoimidazole-4-carboxylic acid (1-az...)Show SMILES Clc1cc(C(=O)NC2CN3CCC2CC3)c2[nH]cnc2c1 |THB:6:7:13.14:11.10,(-1.03,-3.27,;.07,-4.38,;1.41,-3.61,;2.74,-4.39,;4.08,-3.62,;4.09,-2.08,;5.41,-4.39,;6.75,-3.64,;7.8,-4.64,;7.25,-2.85,;8.5,-1.48,;7.53,-.47,;6.32,-1.82,;5.26,-1.62,;5.91,-2.57,;2.74,-5.93,;3.88,-6.97,;3.25,-8.38,;1.71,-8.22,;1.4,-6.7,;.07,-5.91,)| Show InChI InChI=1S/C15H17ClN4O/c16-10-5-11(14-12(6-10)17-8-18-14)15(21)19-13-7-20-3-1-9(13)2-4-20/h5-6,8-9,13H,1-4,7H2,(H,17,18)(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 1195-1198 (1996)
Article DOI: 10.1016/0960-894X(96)00200-4
BindingDB Entry DOI: 10.7270/Q23J3DGF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860
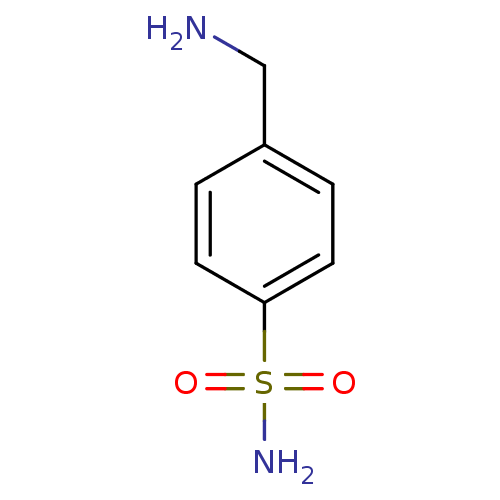
(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860

(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 1195-1198 (1996)
Article DOI: 10.1016/0960-894X(96)00200-4
BindingDB Entry DOI: 10.7270/Q23J3DGF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-inhibitor complex by Lineweaver-Burk d... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate-inhibitor complex by Linewea... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50108392

((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c[nH]c2ccccc12 |r,THB:9:7:4.3:1| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 1195-1198 (1996)
Article DOI: 10.1016/0960-894X(96)00200-4
BindingDB Entry DOI: 10.7270/Q23J3DGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometr... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366063

(CHEMBL1956769)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)N[C@@H]2O[C@H](CO)[C@@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H33N3O12S2/c22-38(32,33)10-3-1-9(2-4-10)5-6-23-21(37)24-19-16(30)15(29)18(12(8-26)34-19)36-20-17(31)14(28)13(27)11(7-25)35-20/h1-4,11-20,25-31H,5-8H2,(H2,22,32,33)(H2,23,24,37)/t11-,12-,13-,14+,15-,16-,17-,18-,19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometric ... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366059
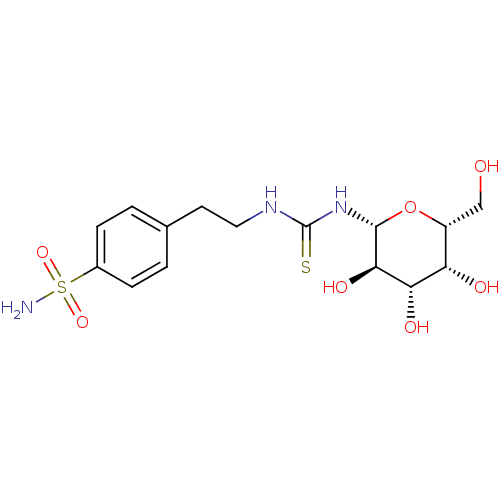
(CHEMBL1956765)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)N[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C15H23N3O7S2/c16-27(23,24)9-3-1-8(2-4-9)5-6-17-15(26)18-14-13(22)12(21)11(20)10(7-19)25-14/h1-4,10-14,19-22H,5-7H2,(H2,16,23,24)(H2,17,18,26)/t10-,11+,12+,13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50278848

(4-(4-{[beta-D-galactopyranosyl]sulfinylmethyl}-1-H...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(CS(=O)[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C15H20N4O8S2/c16-29(25,26)10-3-1-9(2-4-10)19-5-8(17-18-19)7-28(24)15-14(23)13(22)12(21)11(6-20)27-15/h1-5,11-15,20-23H,6-7H2,(H2,16,25,26)/t11-,12+,13+,14-,15+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10861
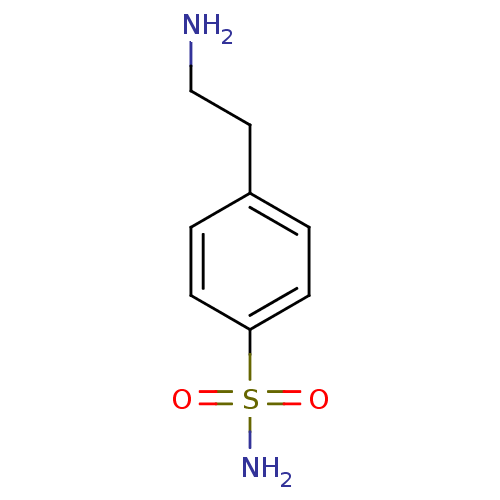
(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10861

(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366076
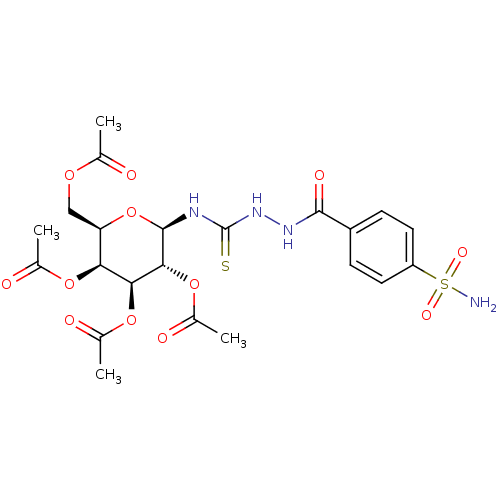
(CHEMBL1956750)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)NNC(=O)c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C22H28N4O12S2/c1-10(27)34-9-16-17(35-11(2)28)18(36-12(3)29)19(37-13(4)30)21(38-16)24-22(39)26-25-20(31)14-5-7-15(8-6-14)40(23,32)33/h5-8,16-19,21H,9H2,1-4H3,(H,25,31)(H2,23,32,33)(H2,24,26,39)/t16-,17+,18+,19-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50083192
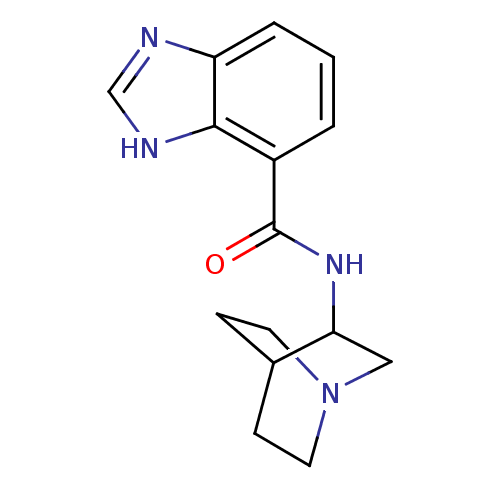
(1H-Benzoimidazole-4-carboxylic acid (1-aza-bicyclo...)Show SMILES O=C(NC1CN2CCC1CC2)c1cccc2nc[nH]c12 |THB:2:3:9.10:7.6,(4.09,-2.08,;4.08,-3.62,;5.41,-4.39,;6.75,-3.64,;7.8,-4.64,;7.25,-2.85,;8.5,-1.48,;7.53,-.47,;6.32,-1.82,;5.26,-1.62,;5.91,-2.57,;2.74,-4.39,;1.41,-3.61,;.07,-4.38,;.07,-5.91,;1.4,-6.7,;1.71,-8.22,;3.25,-8.38,;3.88,-6.97,;2.74,-5.93,)| Show InChI InChI=1S/C15H18N4O/c20-15(11-2-1-3-12-14(11)17-9-16-12)18-13-8-19-6-4-10(13)5-7-19/h1-3,9-10,13H,4-8H2,(H,16,17)(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 1195-1198 (1996)
Article DOI: 10.1016/0960-894X(96)00200-4
BindingDB Entry DOI: 10.7270/Q23J3DGF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain by CO2 hydration assay |
J Med Chem 54: 1481-9 (2011)
Article DOI: 10.1021/jm101525j
BindingDB Entry DOI: 10.7270/Q25T3KSX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA12 catalytic domain by stopped flow CO2 hydration assay |
J Med Chem 52: 6421-32 (2009)
Article DOI: 10.1021/jm900914e
BindingDB Entry DOI: 10.7270/Q2N29XV0 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12918
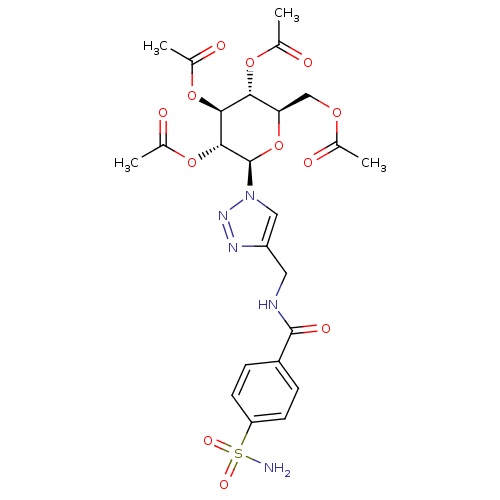
(4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(2,3,...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(CNC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 Show InChI InChI=1S/C24H29N5O12S/c1-12(30)37-11-19-20(38-13(2)31)21(39-14(3)32)22(40-15(4)33)24(41-19)29-10-17(27-28-29)9-26-23(34)16-5-7-18(8-6-16)42(25,35)36/h5-8,10,19-22,24H,9,11H2,1-4H3,(H,26,34)(H2,25,35,36)/t19-,20-,21+,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50314549
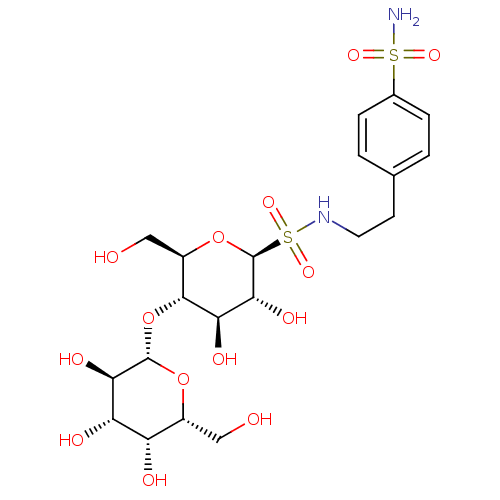
(CHEMBL1090011 | N-4-(Aminosulfonyl)phenethyl-S-(1-...)Show SMILES NS(=O)(=O)c1ccc(CCNS(=O)(=O)[C@@H]2O[C@H](CO)[C@@H](O[C@@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C20H32N2O14S2/c21-37(30,31)10-3-1-9(2-4-10)5-6-22-38(32,33)20-17(29)15(27)18(12(8-24)35-20)36-19-16(28)14(26)13(25)11(7-23)34-19/h1-4,11-20,22-29H,5-8H2,(H2,21,30,31)/t11-,12-,13+,14+,15-,16-,17-,18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50314542
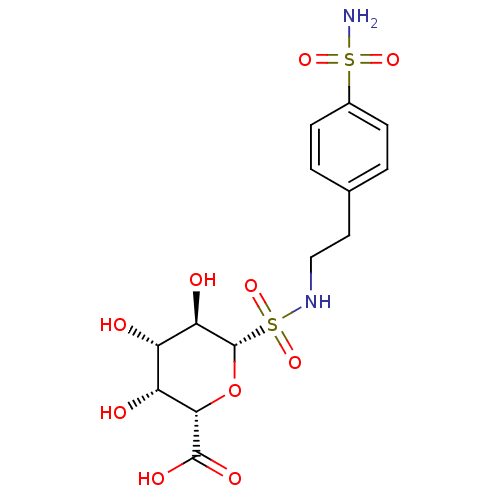
((2S,3R,4S,5R,6S)-3,4,5-trihydroxy-6-(N-(4-sulfamoy...)Show SMILES NS(=O)(=O)c1ccc(CCNS(=O)(=O)[C@@H]2O[C@@H]([C@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C14H20N2O10S2/c15-27(22,23)8-3-1-7(2-4-8)5-6-16-28(24,25)14-11(19)9(17)10(18)12(26-14)13(20)21/h1-4,9-12,14,16-19H,5-6H2,(H,20,21)(H2,15,22,23)/t9-,10+,11+,12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50314547
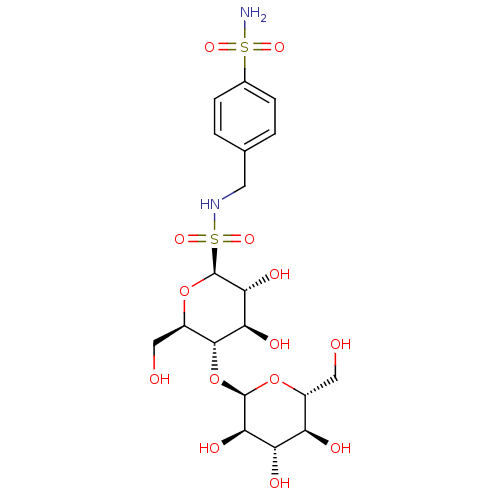
(CHEMBL1089329 | N-4-(Aminosulfonyl)benzyl-S-(1-thi...)Show SMILES NS(=O)(=O)c1ccc(CNS(=O)(=O)[C@@H]2O[C@H](CO)[C@@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C19H30N2O14S2/c20-36(29,30)9-3-1-8(2-4-9)5-21-37(31,32)19-16(28)14(26)17(11(7-23)34-19)35-18-15(27)13(25)12(24)10(6-22)33-18/h1-4,10-19,21-28H,5-7H2,(H2,20,29,30)/t10-,11-,12-,13+,14-,15-,16-,17-,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50083178

(6-Chloro-1H-benzoimidazole-4-carboxylic acid 9-met...)Show SMILES CN1C2CCCC1CC(C2)OC(=O)c1cc(Cl)cc2[nH]cnc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C17H20ClN3O2/c1-21-11-3-2-4-12(21)8-13(7-11)23-17(22)14-5-10(18)6-15-16(14)20-9-19-15/h5-6,9,11-13H,2-4,7-8H2,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 1195-1198 (1996)
Article DOI: 10.1016/0960-894X(96)00200-4
BindingDB Entry DOI: 10.7270/Q23J3DGF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10888
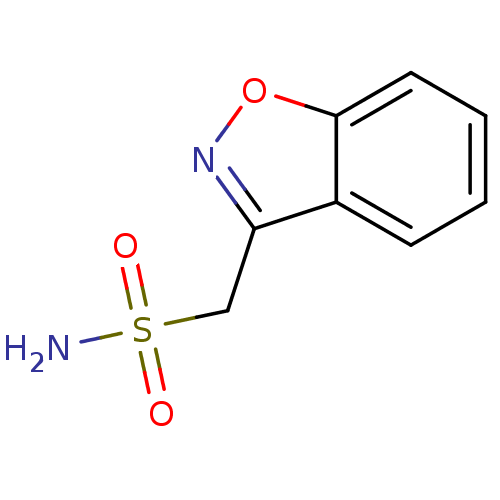
(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA9 catalytic domain by stopped flow CO2 hydration assay |
J Med Chem 52: 6421-32 (2009)
Article DOI: 10.1021/jm900914e
BindingDB Entry DOI: 10.7270/Q2N29XV0 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50314553
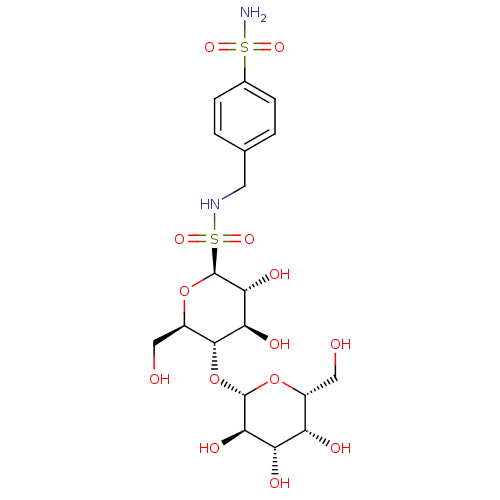
(CHEMBL1088998 | N-4-(Aminosulfonyl)benzyl-S-(1-thi...)Show SMILES NS(=O)(=O)c1ccc(CNS(=O)(=O)[C@@H]2O[C@H](CO)[C@@H](O[C@@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C19H30N2O14S2/c20-36(29,30)9-3-1-8(2-4-9)5-21-37(31,32)19-16(28)14(26)17(11(7-23)34-19)35-18-15(27)13(25)12(24)10(6-22)33-18/h1-4,10-19,21-28H,5-7H2,(H2,20,29,30)/t10-,11-,12+,13+,14-,15-,16-,17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydration assay |
J Med Chem 54: 1481-9 (2011)
Article DOI: 10.1021/jm101525j
BindingDB Entry DOI: 10.7270/Q25T3KSX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by stopped flow CO2 hydration assay |
J Med Chem 52: 6421-32 (2009)
Article DOI: 10.1021/jm900914e
BindingDB Entry DOI: 10.7270/Q2N29XV0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50314526
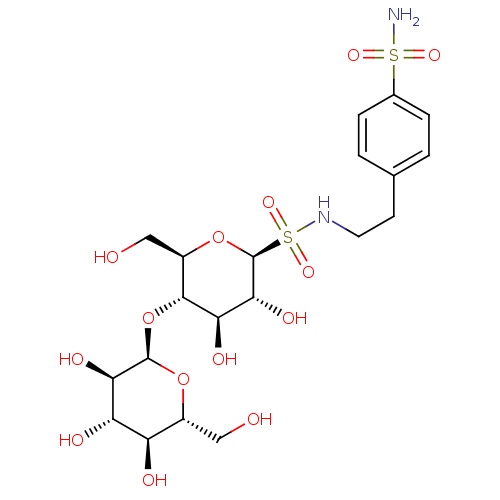
(CHEMBL1090012 | N-4-(Aminosulfonyl)phenethyl-S-(1-...)Show SMILES NS(=O)(=O)c1ccc(CCNS(=O)(=O)[C@@H]2O[C@H](CO)[C@@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C20H32N2O14S2/c21-37(30,31)10-3-1-9(2-4-10)5-6-22-38(32,33)20-17(29)15(27)18(12(8-24)35-20)36-19-16(28)14(26)13(25)11(7-23)34-19/h1-4,11-20,22-29H,5-8H2,(H2,21,30,31)/t11-,12-,13-,14+,15-,16-,17-,18-,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50354340
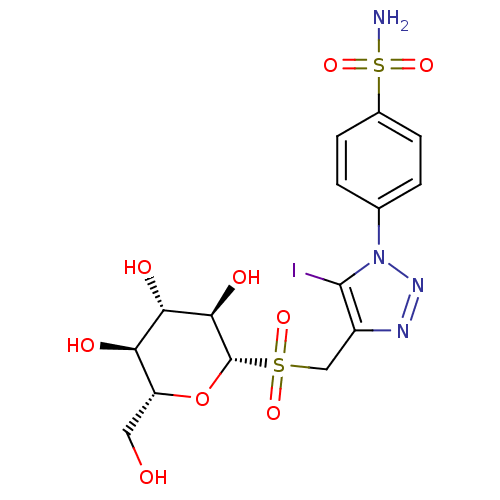
(CHEMBL1836630)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nnc(CS(=O)(=O)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c1I |r| Show InChI InChI=1S/C15H19IN4O9S2/c16-14-9(18-19-20(14)7-1-3-8(4-2-7)31(17,27)28)6-30(25,26)15-13(24)12(23)11(22)10(5-21)29-15/h1-4,10-13,15,21-24H,5-6H2,(H2,17,27,28)/t10-,11-,12+,13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration based stopped flow assay |
J Med Chem 54: 6905-18 (2011)
Article DOI: 10.1021/jm200892s
BindingDB Entry DOI: 10.7270/Q2BG2PDZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
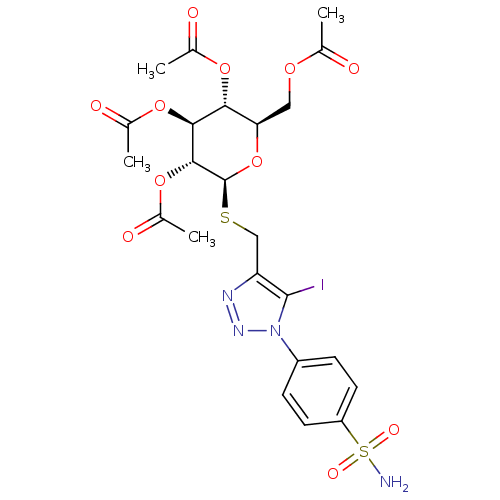
(Homo sapiens (Human)) | BDBM50354347
(CHEMBL1836622)Show SMILES CC(=O)OC[C@H]1O[C@@H](SCc2nnn(c2I)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C23H27IN4O11S2/c1-11(29)35-9-18-19(36-12(2)30)20(37-13(3)31)21(38-14(4)32)23(39-18)40-10-17-22(24)28(27-26-17)15-5-7-16(8-6-15)41(25,33)34/h5-8,18-21,23H,9-10H2,1-4H3,(H2,25,33,34)/t18-,19-,20+,21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by CO2 hydration based stopped flow assay |
J Med Chem 54: 6905-18 (2011)
Article DOI: 10.1021/jm200892s
BindingDB Entry DOI: 10.7270/Q2BG2PDZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
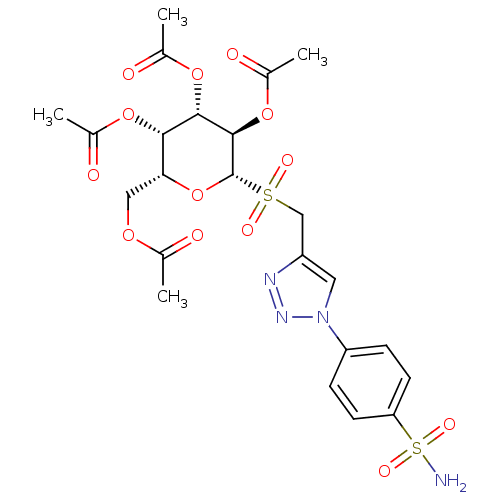
(Homo sapiens (Human)) | BDBM50278783
(4-(4-{[2',3',4',6'-Tetra-O-acetyl-beta-D-galactopy...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O)S(=O)(=O)Cc1cn(nn1)-c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C23H28N4O13S2/c1-12(28)36-10-19-20(37-13(2)29)21(38-14(3)30)22(39-15(4)31)23(40-19)41(32,33)11-16-9-27(26-25-16)17-5-7-18(8-6-17)42(24,34)35/h5-9,19-23H,10-11H2,1-4H3,(H2,24,34,35)/t19-,20+,21+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by CO2 hydration based stopped flow assay |
J Med Chem 54: 6905-18 (2011)
Article DOI: 10.1021/jm200892s
BindingDB Entry DOI: 10.7270/Q2BG2PDZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
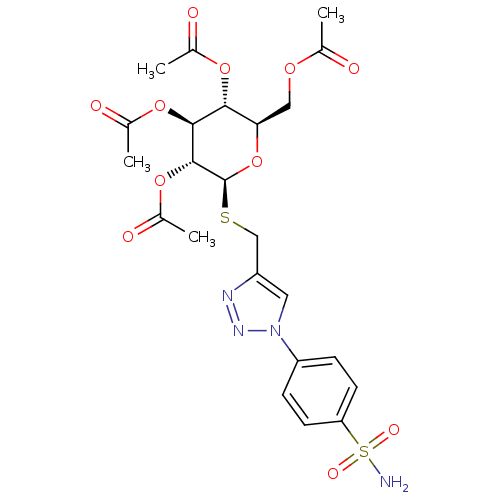
(Homo sapiens (Human)) | BDBM50278700
(4-(4-{[2',3',4',6'-Tetra-O-acetyl-beta-D-glucopyra...)Show SMILES CC(=O)OC[C@H]1O[C@@H](SCc2cn(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C23H28N4O11S2/c1-12(28)34-10-19-20(35-13(2)29)21(36-14(3)30)22(37-15(4)31)23(38-19)39-11-16-9-27(26-25-16)17-5-7-18(8-6-17)40(24,32)33/h5-9,19-23H,10-11H2,1-4H3,(H2,24,32,33)/t19-,20-,21+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by CO2 hydration based stopped flow assay |
J Med Chem 54: 6905-18 (2011)
Article DOI: 10.1021/jm200892s
BindingDB Entry DOI: 10.7270/Q2BG2PDZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50278783

(4-(4-{[2',3',4',6'-Tetra-O-acetyl-beta-D-galactopy...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O)S(=O)(=O)Cc1cn(nn1)-c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C23H28N4O13S2/c1-12(28)36-10-19-20(37-13(2)29)21(38-14(3)30)22(39-15(4)31)23(40-19)41(32,33)11-16-9-27(26-25-16)17-5-7-18(8-6-17)42(24,34)35/h5-9,19-23H,10-11H2,1-4H3,(H2,24,34,35)/t19-,20+,21+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50278700

(4-(4-{[2',3',4',6'-Tetra-O-acetyl-beta-D-glucopyra...)Show SMILES CC(=O)OC[C@H]1O[C@@H](SCc2cn(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C23H28N4O11S2/c1-12(28)34-10-19-20(35-13(2)29)21(36-14(3)30)22(37-15(4)31)23(38-19)39-11-16-9-27(26-25-16)17-5-7-18(8-6-17)40(24,32)33/h5-9,19-23H,10-11H2,1-4H3,(H2,24,32,33)/t19-,20-,21+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50278779
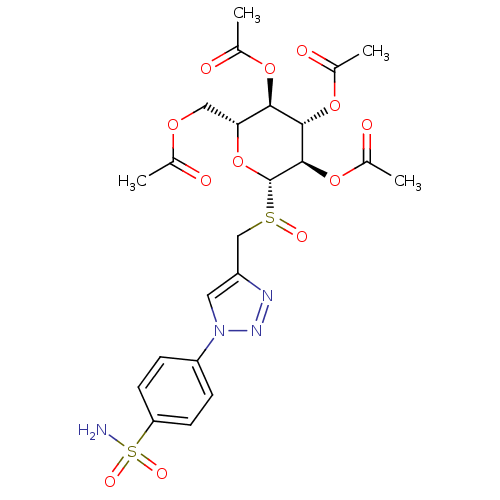
(4-(4-{[2',3',4',6'-Tetra-O-acetyl-beta-D-glucopyra...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)S(=O)Cc1cn(nn1)-c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C23H28N4O12S2/c1-12(28)35-10-19-20(36-13(2)29)21(37-14(3)30)22(38-15(4)31)23(39-19)40(32)11-16-9-27(26-25-16)17-5-7-18(8-6-17)41(24,33)34/h5-9,19-23H,10-11H2,1-4H3,(H2,24,33,34)/t19-,20-,21+,22-,23+,40?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50354334

(CHEMBL1836629)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nnc(CS[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c1I |r| Show InChI InChI=1S/C15H19IN4O7S2/c16-14-9(6-28-15-13(24)12(23)11(22)10(5-21)27-15)18-19-20(14)7-1-3-8(4-2-7)29(17,25)26/h1-4,10-13,15,21-24H,5-6H2,(H2,17,25,26)/t10-,11-,12+,13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by CO2 hydration based stopped flow assay |
J Med Chem 54: 6905-18 (2011)
Article DOI: 10.1021/jm200892s
BindingDB Entry DOI: 10.7270/Q2BG2PDZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
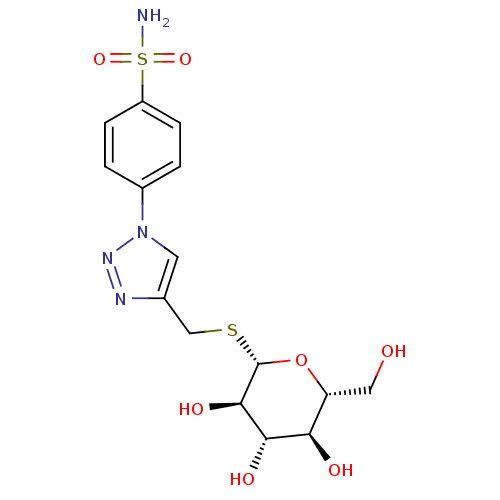
(Homo sapiens (Human)) | BDBM50278844
(4-(4-{[beta-D-glucopyranosyl]thiomethyl}-1-H-1,2,3...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(CS[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C15H20N4O7S2/c16-28(24,25)10-3-1-9(2-4-10)19-5-8(17-18-19)7-27-15-14(23)13(22)12(21)11(6-20)26-15/h1-5,11-15,20-23H,6-7H2,(H2,16,24,25)/t11-,12-,13+,14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50278844

(4-(4-{[beta-D-glucopyranosyl]thiomethyl}-1-H-1,2,3...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(CS[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C15H20N4O7S2/c16-28(24,25)10-3-1-9(2-4-10)19-5-8(17-18-19)7-27-15-14(23)13(22)12(21)11(6-20)26-15/h1-5,11-15,20-23H,6-7H2,(H2,16,24,25)/t11-,12-,13+,14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by CO2 hydration based stopped flow assay |
J Med Chem 54: 6905-18 (2011)
Article DOI: 10.1021/jm200892s
BindingDB Entry DOI: 10.7270/Q2BG2PDZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration based stopped flow assay |
J Med Chem 54: 6905-18 (2011)
Article DOI: 10.1021/jm200892s
BindingDB Entry DOI: 10.7270/Q2BG2PDZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA12 catalytic domain by stopped flow CO2 hydration assay |
J Med Chem 52: 6421-32 (2009)
Article DOI: 10.1021/jm900914e
BindingDB Entry DOI: 10.7270/Q2N29XV0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
Bioorg Med Chem Lett 19: 2273-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.086
BindingDB Entry DOI: 10.7270/Q2QF8TSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366074

(CHEMBL1956748)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)NCc2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C22H29N3O11S2/c1-11(26)32-10-17-18(33-12(2)27)19(34-13(3)28)20(35-14(4)29)21(36-17)25-22(37)24-9-15-5-7-16(8-6-15)38(23,30)31/h5-8,17-21H,9-10H2,1-4H3,(H2,23,30,31)(H2,24,25,37)/t17-,18+,19+,20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50083187
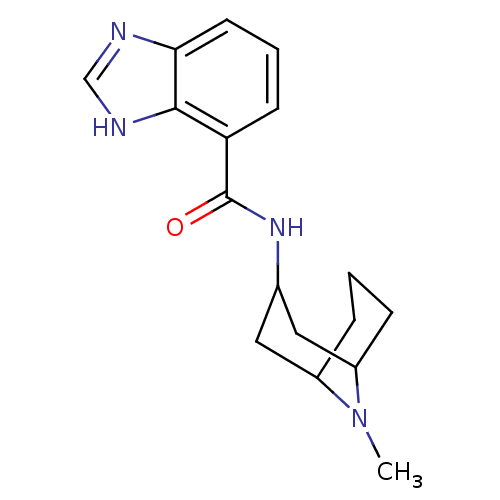
(1H-Benzoimidazole-4-carboxylic acid (9-methyl-9-az...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1cccc2nc[nH]c12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C17H22N4O/c1-21-12-4-2-5-13(21)9-11(8-12)20-17(22)14-6-3-7-15-16(14)19-10-18-15/h3,6-7,10-13H,2,4-5,8-9H2,1H3,(H,18,19)(H,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 1195-1198 (1996)
Article DOI: 10.1016/0960-894X(96)00200-4
BindingDB Entry DOI: 10.7270/Q23J3DGF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data