| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50318708 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_633730 (CHEMBL1119101) |
|---|
| IC50 | 1800±n/a nM |
|---|
| Citation |  de Los Ríos, C; Egea, J; Marco-Contelles, J; León, R; Samadi, A; Iriepa, I; Moraleda, I; Gálvez, E; García, AG; López, MG; Villarroya, M; Romero, A Synthesis, inhibitory activity of cholinesterases, and neuroprotective profile of novel 1,8-naphthyridine derivatives. J Med Chem53:5129-43 (2010) [PubMed] Article de Los Ríos, C; Egea, J; Marco-Contelles, J; León, R; Samadi, A; Iriepa, I; Moraleda, I; Gálvez, E; García, AG; López, MG; Villarroya, M; Romero, A Synthesis, inhibitory activity of cholinesterases, and neuroprotective profile of novel 1,8-naphthyridine derivatives. J Med Chem53:5129-43 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67792.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22303 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPV
SAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSM
NYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASV
GMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTEL
VACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVG
VVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPE
DPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGY
EIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQ
YVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQDRCSDL
|
|
|
|---|
| BDBM50318708 |
|---|
| n/a |
|---|
| Name | BDBM50318708 |
|---|
| Synonyms: | CHEMBL1084517 | Methyl 5-Amino-2-methyl-6,7,8,9,10,11-hexahydrocycloocta-[b][1,8]naphthyridine-3-carboxylate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H21N3O2 |
|---|
| Mol. Mass. | 299.3675 |
|---|
| SMILES | COC(=O)c1cc2c(N)c3CCCCCCc3nc2nc1C |
|---|
| Structure | 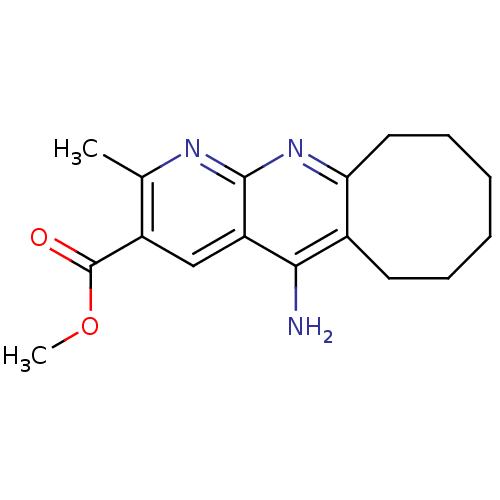
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 de Los Ríos, C; Egea, J; Marco-Contelles, J; León, R; Samadi, A; Iriepa, I; Moraleda, I; Gálvez, E; García, AG; López, MG; Villarroya, M; Romero, A Synthesis, inhibitory activity of cholinesterases, and neuroprotective profile of novel 1,8-naphthyridine derivatives. J Med Chem53:5129-43 (2010) [PubMed] Article
de Los Ríos, C; Egea, J; Marco-Contelles, J; León, R; Samadi, A; Iriepa, I; Moraleda, I; Gálvez, E; García, AG; López, MG; Villarroya, M; Romero, A Synthesis, inhibitory activity of cholinesterases, and neuroprotective profile of novel 1,8-naphthyridine derivatives. J Med Chem53:5129-43 (2010) [PubMed] Article