

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50317074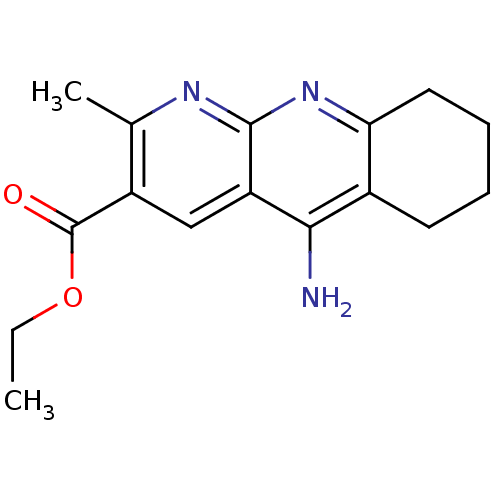 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE (unknown orign) by Lineweaver-Burke plot | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262340 (11-Amino-3,3-dimethyl-12-p-tolyl-3,4,5,7,8,9,10,12...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262341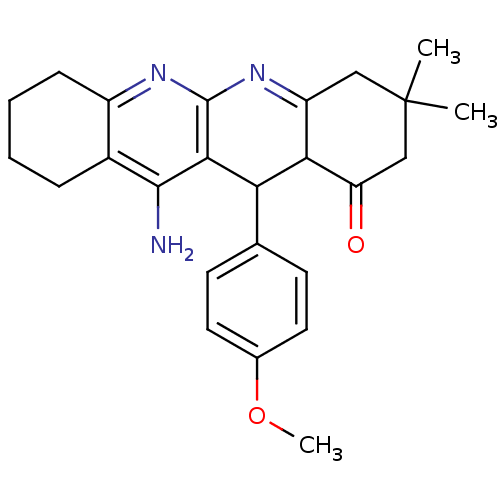 (11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-3,4,5,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262282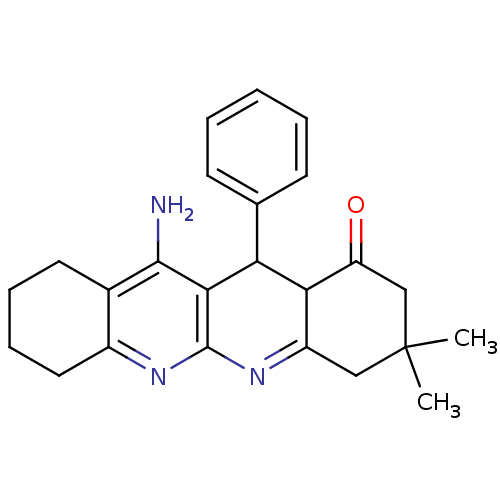 (11-Amino-3,3-dimethyl-12-phenyl-3,4,5,7,8,9,10,12-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262339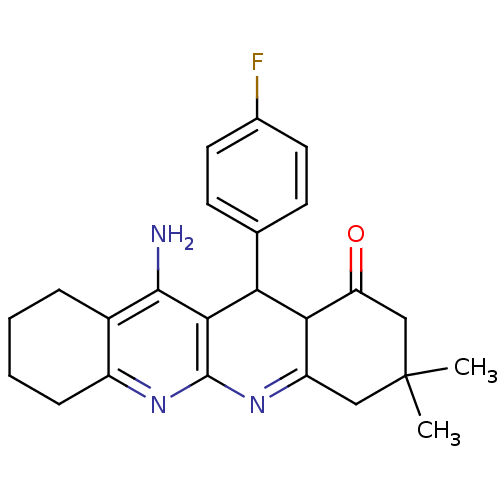 (11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-3,4,5,7...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384793 (CHEMBL2037384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50262342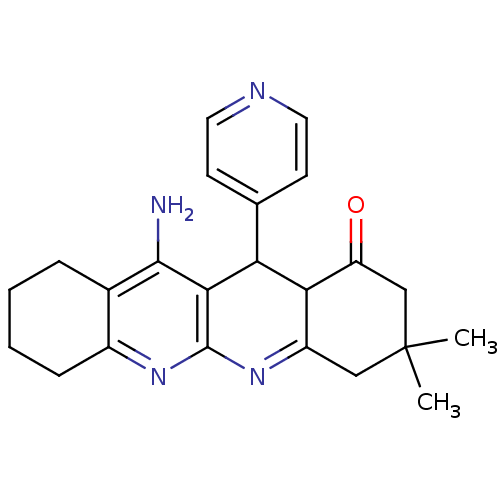 (11-Amino-3,3-dimethyl-12-pyridin-4-yl-3,4,5,7,8,9,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 428 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus AChE by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM31904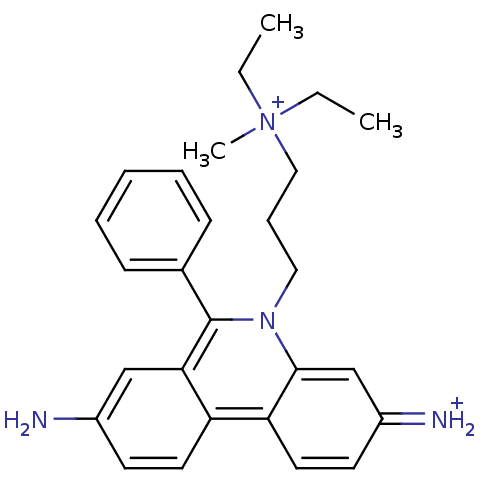 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 16: 7759-69 (2008) Article DOI: 10.1016/j.bmc.2008.07.005 BindingDB Entry DOI: 10.7270/Q2DZ096D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM31904 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Binding affinity to Electrophorus electricus AChE peripheral anionic site | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM31904 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Binding affinity to Electrophorus electricus AChE peripheral anionic site | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50324068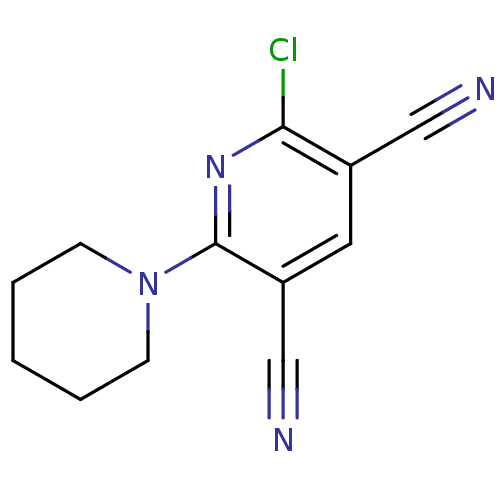 (2-Chloro-6-(piperidin-1-yl)pyridine-3,5-dicarbonit...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Non competitive inhibition of Electrophorus electricus AChE by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50322767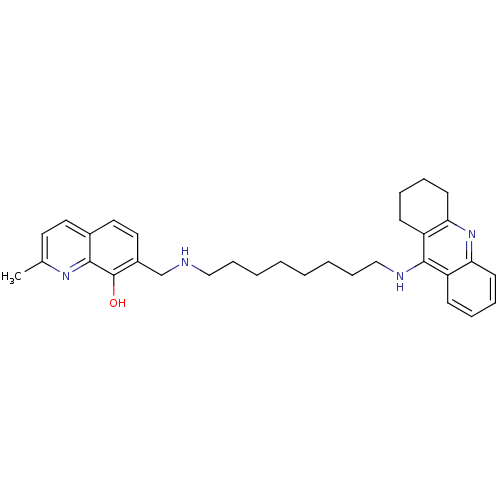 (2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50322768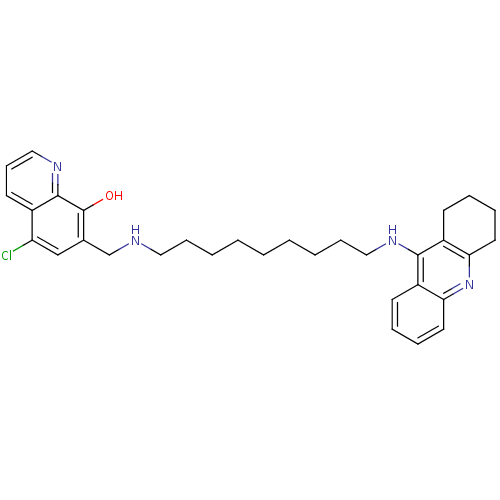 (5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002644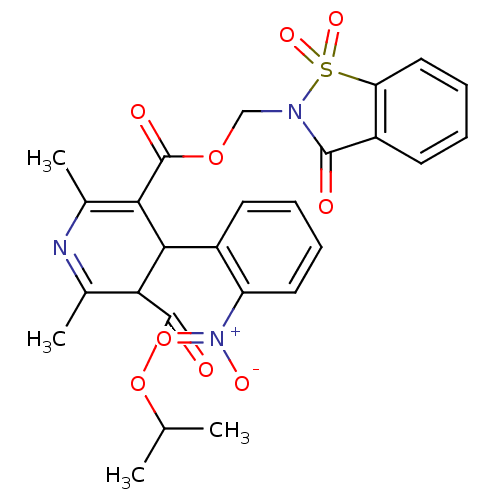 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50322767 (2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002628 (4-(2,3-Dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002603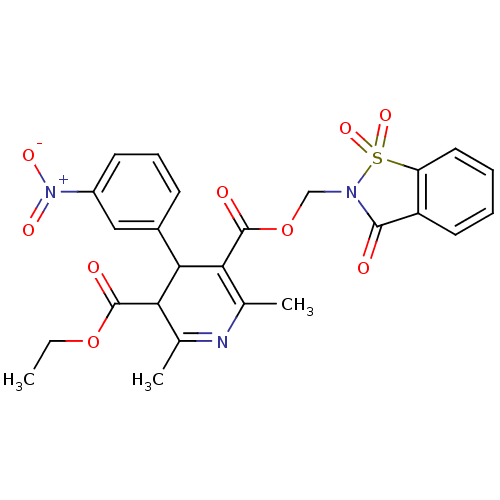 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002613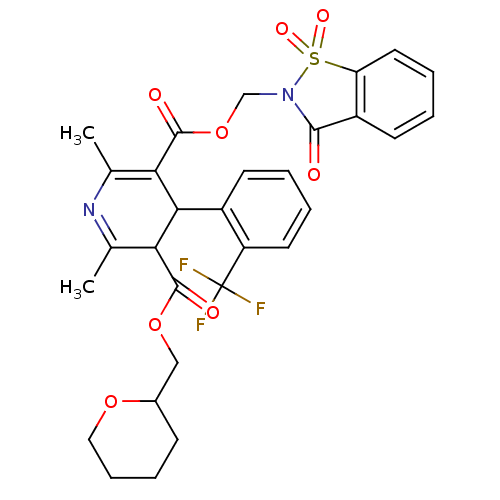 (2,6-Dimethyl-4-(2-trifluoromethyl-phenyl)-1,4-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50322766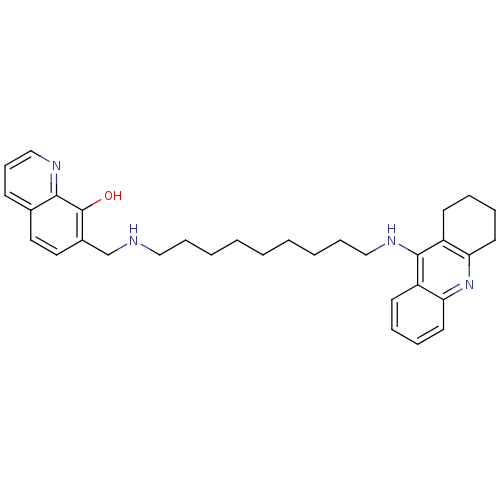 (7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002607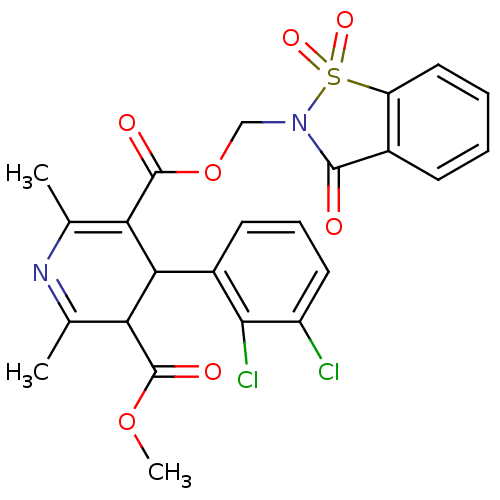 (4-(2,3-Dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002626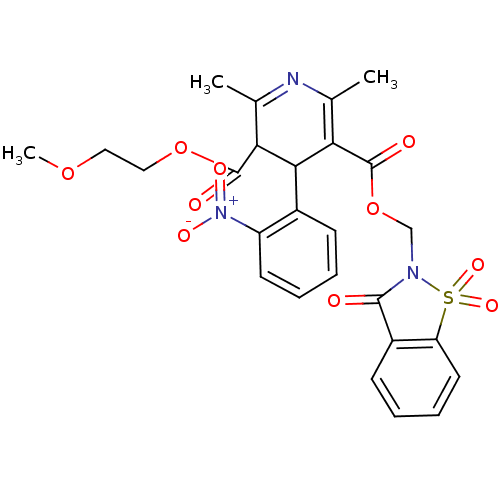 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50322766 (7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50322767 (2-Methyl-7-{[8-(1,2,3,4-tetrahydroacridin-9-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50322768 (5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50322777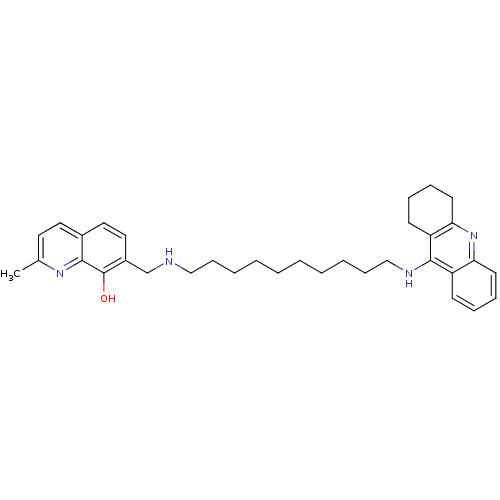 (2-Methyl-7-{[10-(1,2,3,4-tetrahydroacridin-9-ylami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002600 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Eur J Med Chem 45: 6152-8 (2010) Article DOI: 10.1016/j.ejmech.2010.09.039 BindingDB Entry DOI: 10.7270/Q2KW5G84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002643 (4-(2-Chloro-phenyl)-2,6-dimethyl-1,4-dihydro-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum by Ellman method | J Med Chem 52: 7249-57 (2009) Article DOI: 10.1021/jm900628z BindingDB Entry DOI: 10.7270/Q2668DB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002635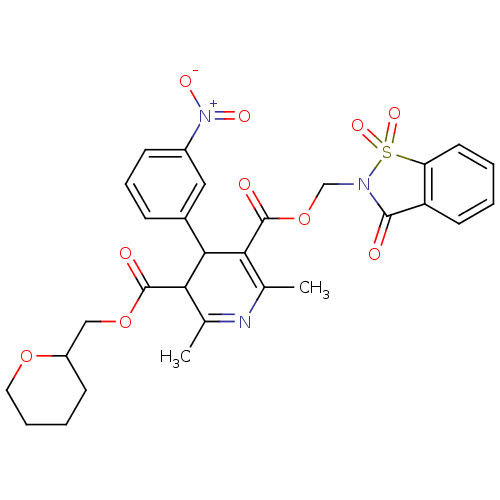 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333148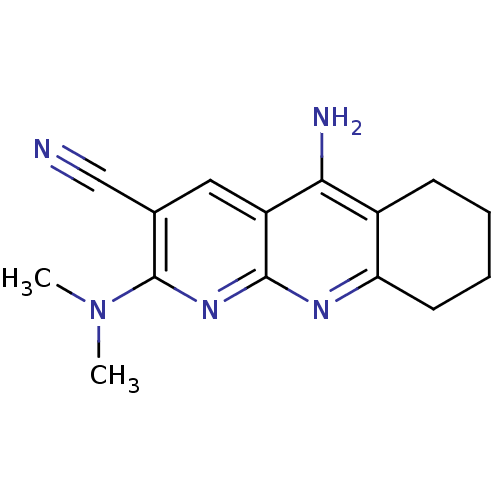 (5-Amino-2-(dimethylamino)-6,7,8,9-tetrahydrobenzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002621 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50322766 (7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50322766 (7-{[9-(1,2,3,4-Tetrahydroacridin-9-ylamino)nonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50322770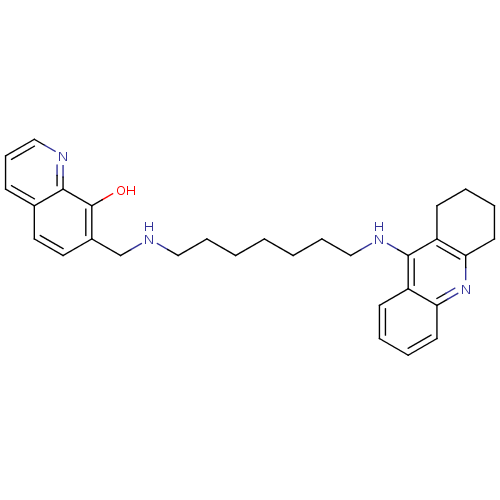 (7-{[7-(1,2,3,4-Tetrahydroacridin-9-ylamino)heptyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's reaction | J Med Chem 53: 4927-37 (2010) Article DOI: 10.1021/jm100329q BindingDB Entry DOI: 10.7270/Q22Z15QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002631 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002580 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333150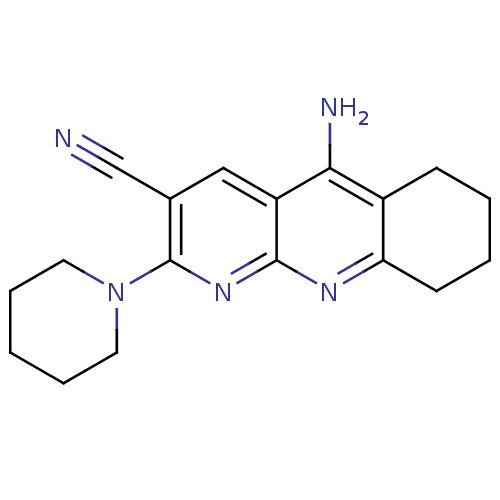 (5-Amino-2-piperidin-1-yl-6,7,8,9-tetrahydrobenzo[1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 376 total ) | Next | Last >> |