| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lysosomal acid glucosylceramidase |
|---|
| Ligand | BDBM50379101 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_809545 (CHEMBL2015462) |
|---|
| pH | 5.2±n/a |
|---|
| Ki | 700±n/a nM |
|---|
| Comments | extracted |
|---|
| Citation |  Trapero, A; Llebaria, A The myo-1,2-Diaminocyclitol Scaffold Defines Potent Glucocerebrosidase Activators and Promising Pharmacological Chaperones for Gaucher Disease. ACS Med Chem Lett2:614-619 (2011) [PubMed] Article Trapero, A; Llebaria, A The myo-1,2-Diaminocyclitol Scaffold Defines Potent Glucocerebrosidase Activators and Promising Pharmacological Chaperones for Gaucher Disease. ACS Med Chem Lett2:614-619 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lysosomal acid glucosylceramidase |
|---|
| Name: | Lysosomal acid glucosylceramidase |
|---|
| Synonyms: | Acid beta-glucosidase | Alglucerase | Beta-glucocerebrosidase | Beta-glucocerebrosidase (GC) | D-glucosyl-N-acylsphingosine glucohydrolase | GBA | GBA1 | GBA1_HUMAN | GC | GCase | GLUC | Glucocerebrosidase (GBA) | Glucosylceramidase (GBA) | Glucosylceramidase (GCase) | Glucosylceramidase precursor (Beta-glucocerebrosidase) (Acid beta-glucosidase) (D-glucosyl-N-acylsphingosine glucohydrolase) (Alglucerase) (Imiglucerase) | Imiglucerase | beta-glucocerebrosidase (GCase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 59724.64 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | The beta-Glu activity was measured with commercially available beta-glucocerebrosidase (Ceredase) as the enzyme source. |
|---|
| Residue: | 536 |
|---|
| Sequence: | MEFSSPSREECPKPLSRVSIMAGSLTGLLLLQAVSWASGARPCIPKSFGYSSVVCVCNAT
YCDSFDPPTFPALGTFSRYESTRSGRRMELSMGPIQANHTGTGLLLTLQPEQKFQKVKGF
GGAMTDAAALNILALSPPAQNLLLKSYFSEEGIGYNIIRVPMASCDFSIRTYTYADTPDD
FQLHNFSLPEEDTKLKIPLIHRALQLAQRPVSLLASPWTSPTWLKTNGAVNGKGSLKGQP
GDIYHQTWARYFVKFLDAYAEHKLQFWAVTAENEPSAGLLSGYPFQCLGFTPEHQRDFIA
RDLGPTLANSTHHNVRLLMLDDQRLLLPHWAKVVLTDPEAAKYVHGIAVHWYLDFLAPAK
ATLGETHRLFPNTMLFASEACVGSKFWEQSVRLGSWDRGMQYSHSIITNLLYHVVGWTDW
NLALNPEGGPNWVRNFVDSPIIVDITKDTFYKQPMFYHLGHFSKFIPEGSQRVGLVASQK
NDLDAVALMHPDGSAVVVVLNRSSKDVPLTIKDPAVGFLETISPGYSIHTYLWRRQ
|
|
|
|---|
| BDBM50379101 |
|---|
| n/a |
|---|
| Name | BDBM50379101 |
|---|
| Synonyms: | CHEMBL2012656 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H48N2O5 |
|---|
| Mol. Mass. | 456.659 |
|---|
| SMILES | CCCCCCCCCN1[C@H]2[C@H]([C@H](O)[C@@H](O)[C@H](O)[C@H]2O)N(CCCCCCCCC)C1=O |r| |
|---|
| Structure | 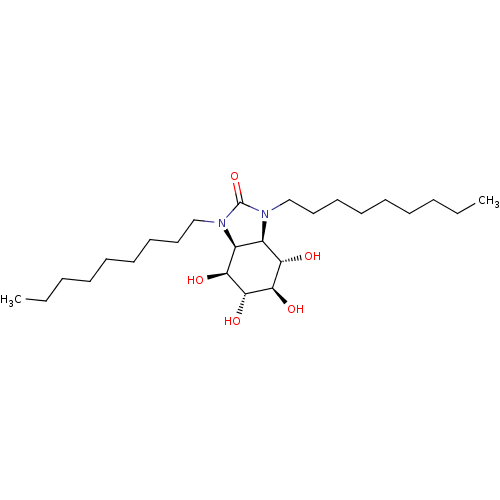
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Trapero, A; Llebaria, A The myo-1,2-Diaminocyclitol Scaffold Defines Potent Glucocerebrosidase Activators and Promising Pharmacological Chaperones for Gaucher Disease. ACS Med Chem Lett2:614-619 (2011) [PubMed] Article
Trapero, A; Llebaria, A The myo-1,2-Diaminocyclitol Scaffold Defines Potent Glucocerebrosidase Activators and Promising Pharmacological Chaperones for Gaucher Disease. ACS Med Chem Lett2:614-619 (2011) [PubMed] Article