 Found 291 hits with Last Name = 'llebaria' and Initial = 'a'
Found 291 hits with Last Name = 'llebaria' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598933
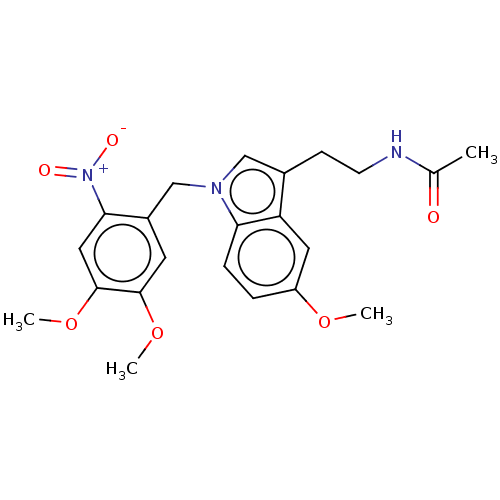
(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394827
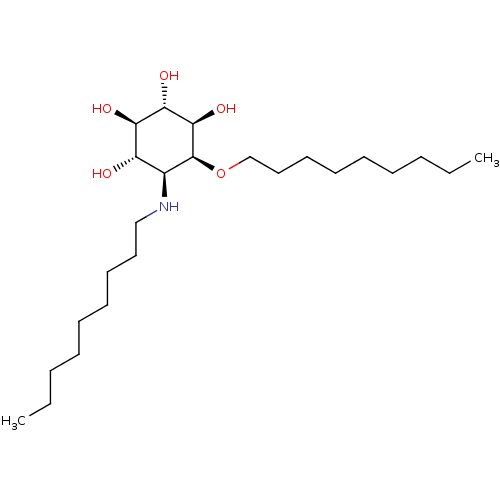
(CHEMBL2164231)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1OCCCCCCCCC |r| Show InChI InChI=1S/C24H49NO5/c1-3-5-7-9-11-13-15-17-25-19-20(26)21(27)22(28)23(29)24(19)30-18-16-14-12-10-8-6-4-2/h19-29H,3-18H2,1-2H3/t19-,20-,21+,22-,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394827

(CHEMBL2164231)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1OCCCCCCCCC |r| Show InChI InChI=1S/C24H49NO5/c1-3-5-7-9-11-13-15-17-25-19-20(26)21(27)22(28)23(29)24(19)30-18-16-14-12-10-8-6-4-2/h19-29H,3-18H2,1-2H3/t19-,20-,21+,22-,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598933

(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598931
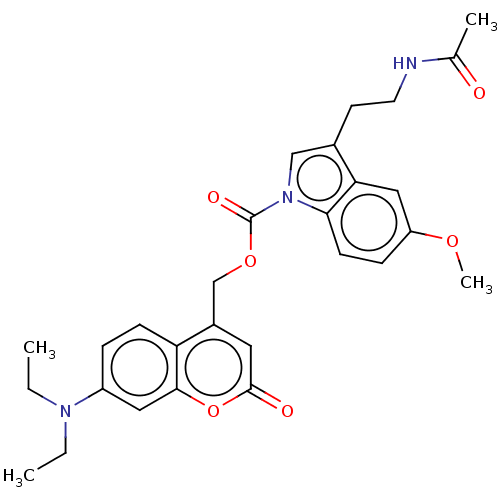
(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598930
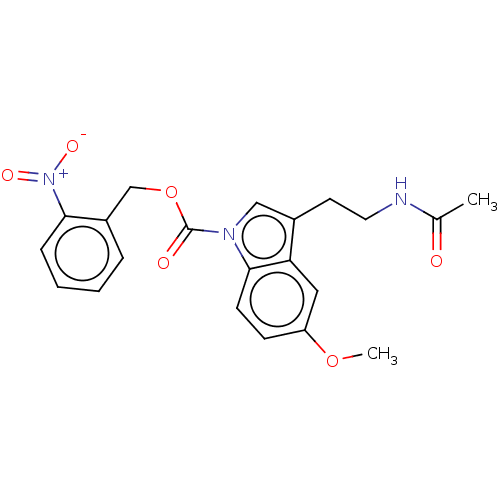
(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598931

(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598930

(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598931

(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598930

(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50379102
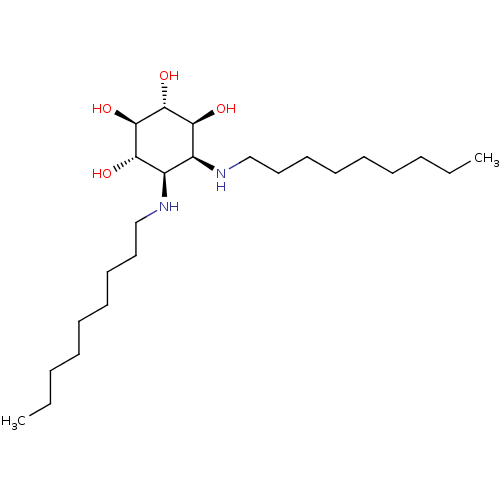
(CHEMBL2012655)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1NCCCCCCCCC |r| Show InChI InChI=1S/C24H50N2O4/c1-3-5-7-9-11-13-15-17-25-19-20(22(28)24(30)23(29)21(19)27)26-18-16-14-12-10-8-6-4-2/h19-30H,3-18H2,1-2H3/t19-,20+,21-,22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant beta-glucocerebrosidase assessed as 4-methyumbelliferone formation after 30 mins by Lineweaver-Burk plot ... |
ACS Med Chem Lett 2: 614-619 (2011)
Article DOI: 10.1021/ml200098j
BindingDB Entry DOI: 10.7270/Q2R78G6C |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394826
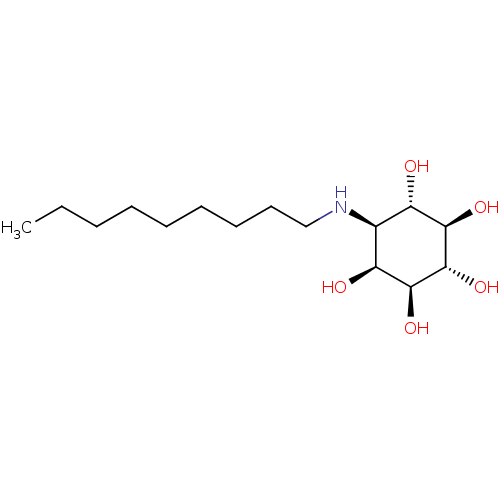
(CHEMBL2164227)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H31NO5/c1-2-3-4-5-6-7-8-9-16-10-11(17)13(19)15(21)14(20)12(10)18/h10-21H,2-9H2,1H3/t10-,11-,12-,13+,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394826

(CHEMBL2164227)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H31NO5/c1-2-3-4-5-6-7-8-9-16-10-11(17)13(19)15(21)14(20)12(10)18/h10-21H,2-9H2,1H3/t10-,11-,12-,13+,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598931

(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50341332
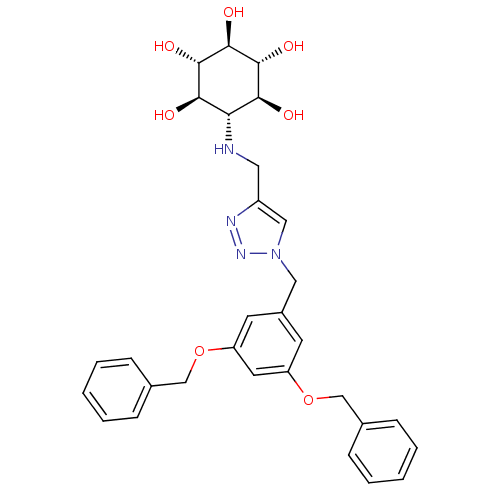
(CHEMBL1766350 | rel-(1R,2S,4R,5S)-6-[(1-(3,5-Bis(b...)Show SMILES O[C@H]1[C@H](O)[C@@H](O)[C@H](NCc2cn(Cc3cc(OCc4ccccc4)cc(OCc4ccccc4)c3)nn2)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C30H34N4O7/c35-26-25(27(36)29(38)30(39)28(26)37)31-14-22-16-34(33-32-22)15-21-11-23(40-17-19-7-3-1-4-8-19)13-24(12-21)41-18-20-9-5-2-6-10-20/h1-13,16,25-31,35-39H,14-15,17-18H2/t25-,26-,27+,28+,29-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50341324

(CHEMBL1766482 | rel-(1R,2S,4R,5S)-6-[[1-Undecyl-1H...)Show SMILES CCCCCCCCCCCn1cc(CN[C@@H]2[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C20H38N4O5/c1-2-3-4-5-6-7-8-9-10-11-24-13-14(22-23-24)12-21-15-16(25)18(27)20(29)19(28)17(15)26/h13,15-21,25-29H,2-12H2,1H3/t15-,16-,17+,18+,19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394825
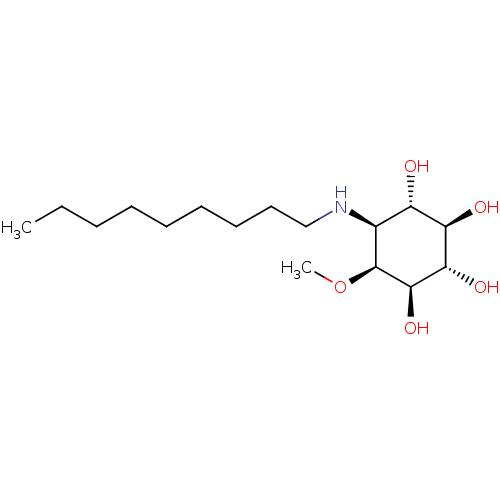
(CHEMBL2164230)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1OC |r| Show InChI InChI=1S/C16H33NO5/c1-3-4-5-6-7-8-9-10-17-11-12(18)13(19)14(20)15(21)16(11)22-2/h11-21H,3-10H2,1-2H3/t11-,12-,13+,14-,15+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394825

(CHEMBL2164230)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1OC |r| Show InChI InChI=1S/C16H33NO5/c1-3-4-5-6-7-8-9-10-17-11-12(18)13(19)14(20)15(21)16(11)22-2/h11-21H,3-10H2,1-2H3/t11-,12-,13+,14-,15+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50318564
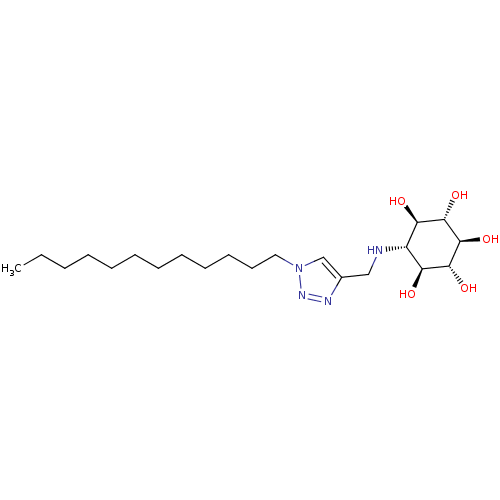
((1R,2S,3r,4R,5S,6s)-6-((1-dodecyl-1H-1,2,3-triazol...)Show SMILES CCCCCCCCCCCCn1cc(CN[C@@H]2[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C21H40N4O5/c1-2-3-4-5-6-7-8-9-10-11-12-25-14-15(23-24-25)13-22-16-17(26)19(28)21(30)20(29)18(16)27/h14,16-22,26-30H,2-13H2,1H3/t16-,17-,18+,19+,20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis |
J Med Chem 53: 5248-55 (2010)
Article DOI: 10.1021/jm100198t
BindingDB Entry DOI: 10.7270/Q2VH5PS1 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50318563
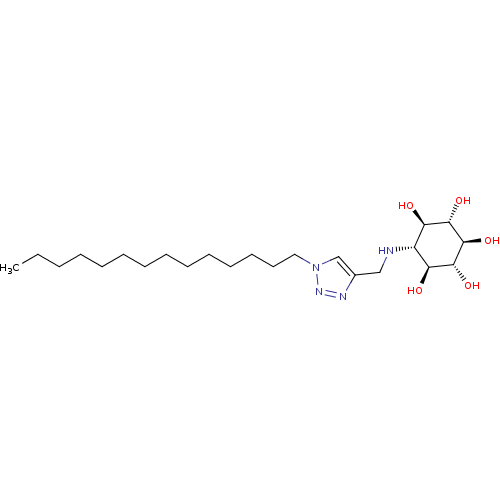
((1R,2S,3r,4R,5S,6s)-6-[(1-Tetradecyl-1H-1,2,3-tria...)Show SMILES CCCCCCCCCCCCCCn1cc(CN[C@@H]2[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C23H44N4O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-27-16-17(25-26-27)15-24-18-19(28)21(30)23(32)22(31)20(18)29/h16,18-24,28-32H,2-15H2,1H3/t18-,19-,20+,21+,22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis |
J Med Chem 53: 5248-55 (2010)
Article DOI: 10.1021/jm100198t
BindingDB Entry DOI: 10.7270/Q2VH5PS1 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50318562

((1R,2S,3r,4R,5S,6s)-6-[(1-Decyl-1H-1,2,3-triazol-4...)Show SMILES CCCCCCCCCCn1cc(CN[C@@H]2[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C19H36N4O5/c1-2-3-4-5-6-7-8-9-10-23-12-13(21-22-23)11-20-14-15(24)17(26)19(28)18(27)16(14)25/h12,14-20,24-28H,2-11H2,1H3/t14-,15-,16+,17+,18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis |
J Med Chem 53: 5248-55 (2010)
Article DOI: 10.1021/jm100198t
BindingDB Entry DOI: 10.7270/Q2VH5PS1 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394824
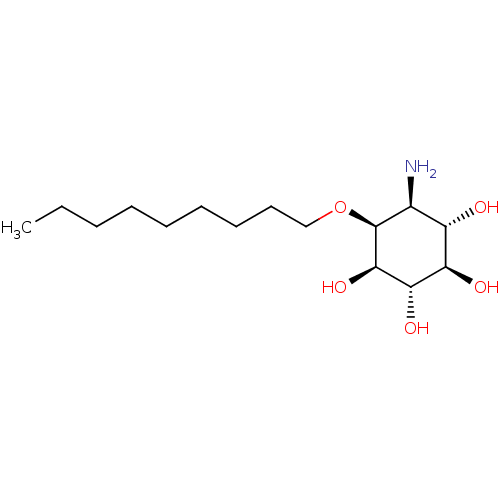
(CHEMBL2164232)Show SMILES CCCCCCCCCO[C@H]1[C@@H](N)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H31NO5/c1-2-3-4-5-6-7-8-9-21-15-10(16)11(17)12(18)13(19)14(15)20/h10-15,17-20H,2-9,16H2,1H3/t10-,11-,12+,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394824

(CHEMBL2164232)Show SMILES CCCCCCCCCO[C@H]1[C@@H](N)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H31NO5/c1-2-3-4-5-6-7-8-9-21-15-10(16)11(17)12(18)13(19)14(15)20/h10-15,17-20H,2-9,16H2,1H3/t10-,11-,12+,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598930

(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50379103
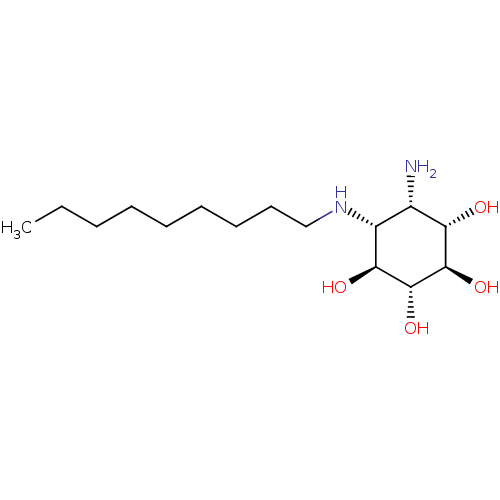
(CHEMBL2012654)Show SMILES CCCCCCCCCN[C@@H]1[C@H](N)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H32N2O4/c1-2-3-4-5-6-7-8-9-17-11-10(16)12(18)14(20)15(21)13(11)19/h10-15,17-21H,2-9,16H2,1H3/t10-,11+,12-,13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant beta-glucocerebrosidase assessed as 4-methyumbelliferone formation after 30 mins by Lineweaver-Burk plot ... |
ACS Med Chem Lett 2: 614-619 (2011)
Article DOI: 10.1021/ml200098j
BindingDB Entry DOI: 10.7270/Q2R78G6C |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598933

(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394823
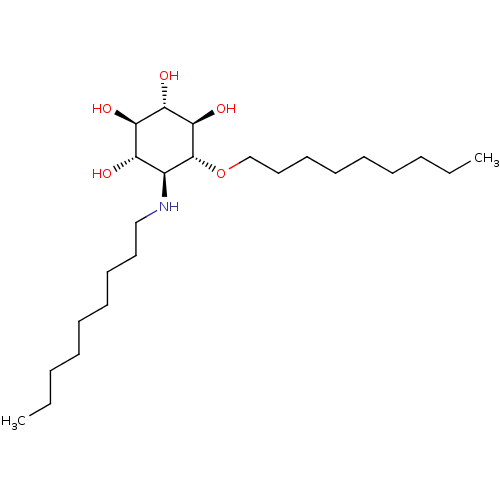
(CHEMBL2164229)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OCCCCCCCCC |r| Show InChI InChI=1S/C24H49NO5/c1-3-5-7-9-11-13-15-17-25-19-20(26)21(27)22(28)23(29)24(19)30-18-16-14-12-10-8-6-4-2/h19-29H,3-18H2,1-2H3/t19-,20-,21+,22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394823

(CHEMBL2164229)Show SMILES CCCCCCCCCN[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OCCCCCCCCC |r| Show InChI InChI=1S/C24H49NO5/c1-3-5-7-9-11-13-15-17-25-19-20(26)21(27)22(28)23(29)24(19)30-18-16-14-12-10-8-6-4-2/h19-29H,3-18H2,1-2H3/t19-,20-,21+,22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50341335
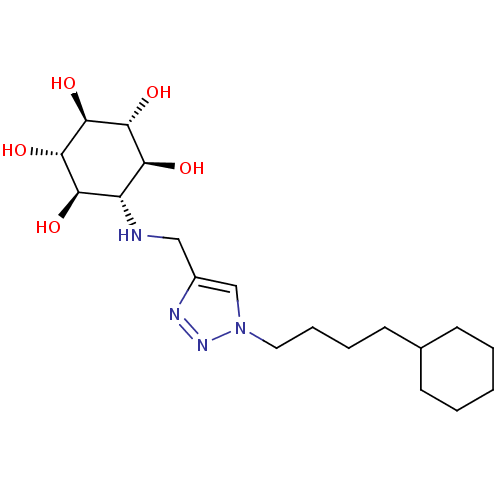
(CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...)Show SMILES O[C@H]1[C@H](O)[C@@H](O)[C@H](NCc2cn(CCCCC3CCCCC3)nn2)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C19H34N4O5/c24-15-14(16(25)18(27)19(28)17(15)26)20-10-13-11-23(22-21-13)9-5-4-8-12-6-2-1-3-7-12/h11-12,14-20,24-28H,1-10H2/t14-,15-,16+,17+,18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18358
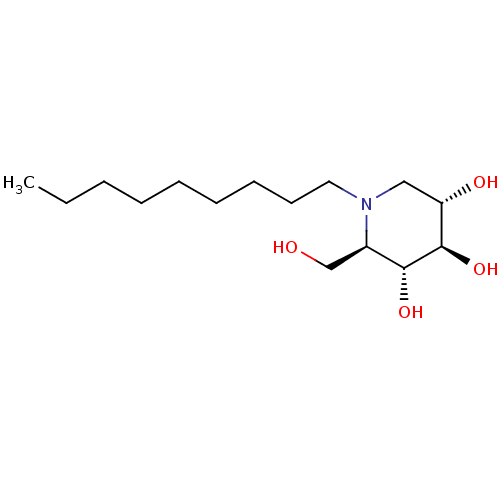
((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...)Show SMILES CCCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C15H31NO4/c1-2-3-4-5-6-7-8-9-16-10-13(18)15(20)14(19)12(16)11-17/h12-15,17-20H,2-11H2,1H3/t12-,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis |
J Med Chem 53: 5248-55 (2010)
Article DOI: 10.1021/jm100198t
BindingDB Entry DOI: 10.7270/Q2VH5PS1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50318560

(CHEMBL1083795 | N-decylaminocyclitol)Show SMILES CCCCCCCCCCN[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H33NO5/c1-2-3-4-5-6-7-8-9-10-17-11-12(18)14(20)16(22)15(21)13(11)19/h11-22H,2-10H2,1H3/t11-,12-,13+,14+,15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis |
J Med Chem 53: 5248-55 (2010)
Article DOI: 10.1021/jm100198t
BindingDB Entry DOI: 10.7270/Q2VH5PS1 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18358

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...)Show SMILES CCCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C15H31NO4/c1-2-3-4-5-6-7-8-9-16-10-13(18)15(20)14(19)12(16)11-17/h12-15,17-20H,2-11H2,1H3/t12-,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18358

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...)Show SMILES CCCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C15H31NO4/c1-2-3-4-5-6-7-8-9-16-10-13(18)15(20)14(19)12(16)11-17/h12-15,17-20H,2-11H2,1H3/t12-,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50318560

(CHEMBL1083795 | N-decylaminocyclitol)Show SMILES CCCCCCCCCCN[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H33NO5/c1-2-3-4-5-6-7-8-9-10-17-11-12(18)14(20)16(22)15(21)13(11)19/h11-22H,2-10H2,1H3/t11-,12-,13+,14+,15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18358

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...)Show SMILES CCCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C15H31NO4/c1-2-3-4-5-6-7-8-9-16-10-13(18)15(20)14(19)12(16)11-17/h12-15,17-20H,2-11H2,1H3/t12-,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18358

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...)Show SMILES CCCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C15H31NO4/c1-2-3-4-5-6-7-8-9-16-10-13(18)15(20)14(19)12(16)11-17/h12-15,17-20H,2-11H2,1H3/t12-,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant beta-glucocerebrosidase assessed as 4-methyumbelliferone formation after 30 mins by Lineweaver-Burk plot ... |
ACS Med Chem Lett 2: 614-619 (2011)
Article DOI: 10.1021/ml200098j
BindingDB Entry DOI: 10.7270/Q2R78G6C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50318561
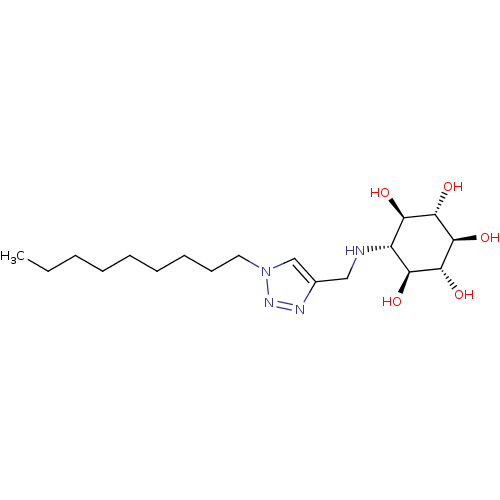
((1R,2S,3r,4R,5S,6s)-6-[(1-Nonyl-1H-1,2,3-triazol-4...)Show SMILES CCCCCCCCCn1cc(CN[C@@H]2[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C18H34N4O5/c1-2-3-4-5-6-7-8-9-22-11-12(20-21-22)10-19-13-14(23)16(25)18(27)17(26)15(13)24/h11,13-19,23-27H,2-10H2,1H3/t13-,14-,15+,16+,17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis |
J Med Chem 53: 5248-55 (2010)
Article DOI: 10.1021/jm100198t
BindingDB Entry DOI: 10.7270/Q2VH5PS1 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598933

(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50379101
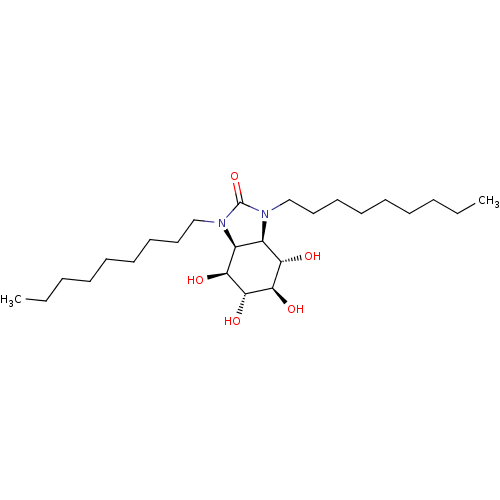
(CHEMBL2012656)Show SMILES CCCCCCCCCN1[C@H]2[C@H]([C@H](O)[C@@H](O)[C@H](O)[C@H]2O)N(CCCCCCCCC)C1=O |r| Show InChI InChI=1S/C25H48N2O5/c1-3-5-7-9-11-13-15-17-26-19-20(22(29)24(31)23(30)21(19)28)27(25(26)32)18-16-14-12-10-8-6-4-2/h19-24,28-31H,3-18H2,1-2H3/t19-,20+,21-,22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant beta-glucocerebrosidase assessed as 4-methyumbelliferone formation after 30 mins by Lineweaver-Burk plot ... |
ACS Med Chem Lett 2: 614-619 (2011)
Article DOI: 10.1021/ml200098j
BindingDB Entry DOI: 10.7270/Q2R78G6C |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50341333
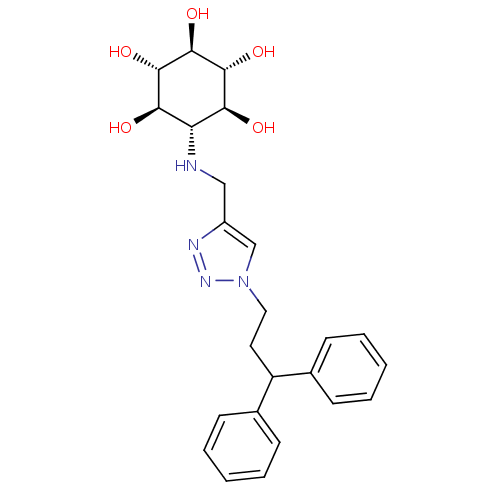
(CHEMBL1766470 | rel-(1R,2S,4R,5S)-6-[[1-(3,3-Diphe...)Show SMILES O[C@H]1[C@H](O)[C@@H](O)[C@H](NCc2cn(CCC(c3ccccc3)c3ccccc3)nn2)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C24H30N4O5/c29-20-19(21(30)23(32)24(33)22(20)31)25-13-17-14-28(27-26-17)12-11-18(15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,14,18-25,29-33H,11-13H2/t19-,20-,21+,22+,23-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | |
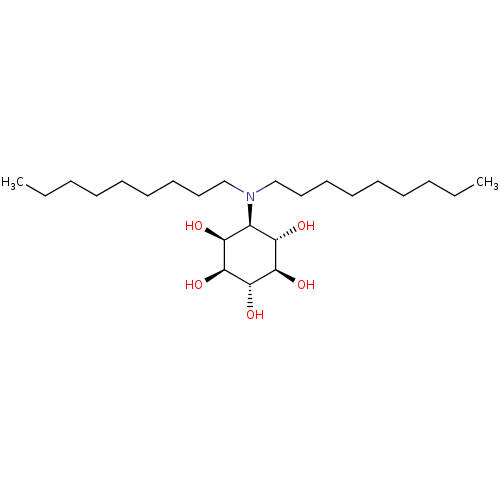
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394831
(CHEMBL2164233)Show SMILES CCCCCCCCCN(CCCCCCCCC)[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H49NO5/c1-3-5-7-9-11-13-15-17-25(18-16-14-12-10-8-6-4-2)19-20(26)22(28)24(30)23(29)21(19)27/h19-24,26-30H,3-18H2,1-2H3/t19-,20-,21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50394831

(CHEMBL2164233)Show SMILES CCCCCCCCCN(CCCCCCCCC)[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H49NO5/c1-3-5-7-9-11-13-15-17-25(18-16-14-12-10-8-6-4-2)19-20(26)22(28)24(30)23(29)21(19)27/h19-24,26-30H,3-18H2,1-2H3/t19-,20-,21-,22+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC)
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... |
J Med Chem 55: 4479-88 (2012)
Article DOI: 10.1021/jm300342q
BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50341331

(CHEMBL1766489 | rel-(1R,2S,4R,5S)-6-[[1-(10-Hydrox...)Show SMILES OCCCCCCCCCCn1cc(CN[C@@H]2[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C19H36N4O6/c24-10-8-6-4-2-1-3-5-7-9-23-12-13(21-22-23)11-20-14-15(25)17(27)19(29)18(28)16(14)26/h12,14-20,24-29H,1-11H2/t14-,15-,16+,17+,18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50341334
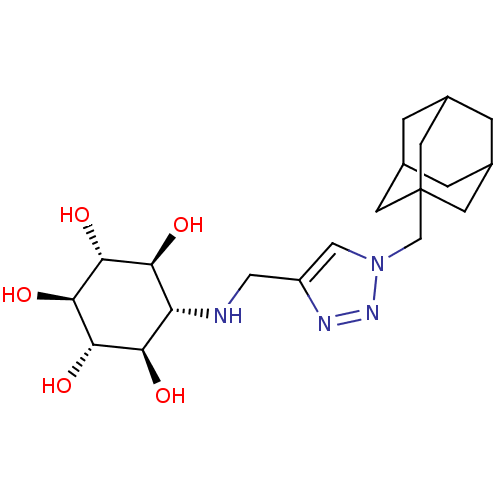
(CHEMBL1766473 | rel-(1R,2S,4R,5S)-6-[(1-Adamantyl-...)Show SMILES O[C@H]1[C@H](O)[C@@H](O)[C@H](NCc2cn(CC34CC5CC(CC(C5)C3)C4)nn2)[C@@H](O)[C@@H]1O |r,TLB:12:13:16:20.19.18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16.15.20:18| Show InChI InChI=1S/C20H32N4O5/c25-15-14(16(26)18(28)19(29)17(15)27)21-7-13-8-24(23-22-13)9-20-4-10-1-11(5-20)3-12(2-10)6-20/h8,10-12,14-19,21,25-29H,1-7,9H2/t10?,11?,12?,14-,15-,16+,17+,18-,19-,20? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas)
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant glucocerebrosidase |
J Med Chem 54: 2069-79 (2011)
Article DOI: 10.1021/jm101204u
BindingDB Entry DOI: 10.7270/Q24F1R18 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data