| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H3 receptor |
|---|
| Ligand | BDBM50383459 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_818296 (CHEMBL2034344) |
|---|
| Ki | 2.6±n/a nM |
|---|
| Citation |  Xiao, D; Palani, A; Sofolarides, M; Aslanian, R; West, RE; Williams, SM; Wu, RL; Hwa, J; Sondey, C; Lachowicz, J; Korfmacher, WA Fused bicycles as arylketone bioisosteres leading to potent, orally active thiadiazole H3 antagonists. Bioorg Med Chem Lett22:3354-7 (2012) [PubMed] Article Xiao, D; Palani, A; Sofolarides, M; Aslanian, R; West, RE; Williams, SM; Wu, RL; Hwa, J; Sondey, C; Lachowicz, J; Korfmacher, WA Fused bicycles as arylketone bioisosteres leading to potent, orally active thiadiazole H3 antagonists. Bioorg Med Chem Lett22:3354-7 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H3 receptor |
|---|
| Name: | Histamine H3 receptor |
|---|
| Synonyms: | HH3R | HRH3_MOUSE | Hrh3 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 48560.37 |
|---|
| Organism: | Mus musculus |
|---|
| Description: | ChEMBL_988451 |
|---|
| Residue: | 445 |
|---|
| Sequence: | MERAPPDGLMNASGALAGEAAAAGGARGFSAAWTAVLAALMALLIVATVLGNALVMLAFV
ADSSLRTQNNFFLLNLAISDFLVGAFCIPLYVPYVLTGRWTFGRGLCKLWLVVDYLLCAS
SVFNIVLISYDRFLSVTRAVSYRAQQGDTRRAVRKMALVWVLAFLLYGPAILSWEYLSGG
SSIPEGHCYAEFFYNWYFLITASTLEFFTPFLSVTFFNLSIYLNIQRRTRLRLDGGREAG
PEPPPDAQPSPPPAPPSCWGCWPKGHGEAMPLHRYGVGEAGPGVETGEAGLGGGSGGGAA
ASPTSSSGSSSRGTERPRSLKRGSKPSASSASLEKRMKMVSQSITQRFRLSRDKKVAKSL
AIIVSIFGLCWAPYTLLMIIRAACHGHCVPDYWYETSFWLLWANSAVNPVLYPLCHYSFR
RAFTKLLCPQKLKVQPHGSLEQCWK
|
|
|
|---|
| BDBM50383459 |
|---|
| n/a |
|---|
| Name | BDBM50383459 |
|---|
| Synonyms: | CHEMBL2031619 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H24N6OS |
|---|
| Mol. Mass. | 396.509 |
|---|
| SMILES | O=c1ccnc2c(cccn12)-c1nnc(s1)N1CCC(CC1)N1CCCCC1 |
|---|
| Structure | 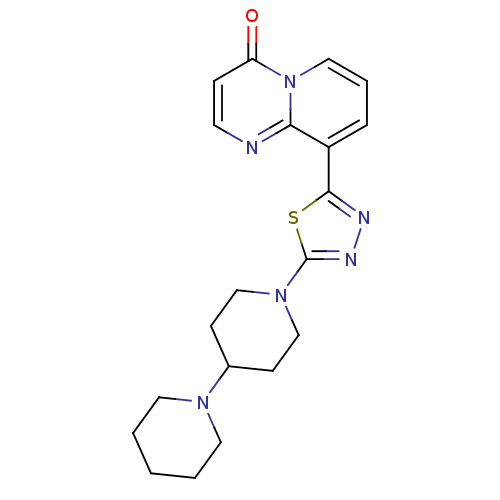
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xiao, D; Palani, A; Sofolarides, M; Aslanian, R; West, RE; Williams, SM; Wu, RL; Hwa, J; Sondey, C; Lachowicz, J; Korfmacher, WA Fused bicycles as arylketone bioisosteres leading to potent, orally active thiadiazole H3 antagonists. Bioorg Med Chem Lett22:3354-7 (2012) [PubMed] Article
Xiao, D; Palani, A; Sofolarides, M; Aslanian, R; West, RE; Williams, SM; Wu, RL; Hwa, J; Sondey, C; Lachowicz, J; Korfmacher, WA Fused bicycles as arylketone bioisosteres leading to potent, orally active thiadiazole H3 antagonists. Bioorg Med Chem Lett22:3354-7 (2012) [PubMed] Article