| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Carboxylic ester hydrolase |
|---|
| Ligand | BDBM10622 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_829364 (CHEMBL2060155) |
|---|
| IC50 | 30000±n/a nM |
|---|
| Citation |  Shinada, M; Narumi, F; Osada, Y; Matsumoto, K; Yoshida, T; Higuchi, K; Kawasaki, T; Tanaka, H; Satoh, M Synthesis of phenserine analogues and evaluation of their cholinesterase inhibitory activities. Bioorg Med Chem20:4901-14 (2012) [PubMed] Article Shinada, M; Narumi, F; Osada, Y; Matsumoto, K; Yoshida, T; Higuchi, K; Kawasaki, T; Tanaka, H; Satoh, M Synthesis of phenserine analogues and evaluation of their cholinesterase inhibitory activities. Bioorg Med Chem20:4901-14 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Carboxylic ester hydrolase |
|---|
| Name: | Carboxylic ester hydrolase |
|---|
| Synonyms: | Acetylcholinesterase and butyrylcholinesterase (AChE and BChE) | BuChE | Butyrylcholinesterase (BuChE) | butyrylcholinesterase precursor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67776.22 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | n/a |
|---|
| Residue: | 597 |
|---|
| Sequence: | MVTEIHFLLWILLLCMLFGKSHTEEDVIITTKTGRVRGLSMPILGGTVTAFLGIPYAQPP
LGSLRFKKPQPLNKWPDVYNATKYANSCYQNIDQAFPGFQGSEMWNPNTNLSEDCLYLNV
WIPVPKPKNATVMVWVYGGGFQTGTSSLPVYDGKFLTRVERVIVVSMNYRVGALGFLAFP
GNSEAPGNMGLFDQQLALQWIQRNIAAFGGNPKSVTLFGESAGAASVSLHLLCPQSYPLF
TRAILESGSSNAPWAVKHPEEARNRTLTLAKFIGCSKENEKEIITCLRSKDPQEILLNEK
LVLPSDSIRSINFGPTVDGDFLTDMPHTLLQLGKVKTAQILVGVNKDEGTAFLVYGAPGF
SKDNDSLITRREFQEGLNMYFPGVSSLGKEAILFYYVDWLGDQTPEVYREAFDDIIGDYN
IICPALEFTKKFAELEINAFFYYFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLERRVNYT
RAEEIFSRSIMKTWANFAKYGHPNGTQGNSTVWPVFTSTEQKYLTLNTEKSKINSKLRAP
QCQFWRLFFPKVLEITGDIDEREQEWKAGFHRWSNYMMDWKNQFNDYTSKKETCTDL
|
|
|
|---|
| BDBM10622 |
|---|
| n/a |
|---|
| Name | BDBM10622 |
|---|
| Synonyms: | (-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-5-yl N-phenylcarbamate | CHEMBL54727 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H23N3O2 |
|---|
| Mol. Mass. | 337.4155 |
|---|
| SMILES | CN1CC[C@]2(C)C1N(C)c1ccc(OC(=O)Nc3ccccc3)cc21 |r| |
|---|
| Structure | 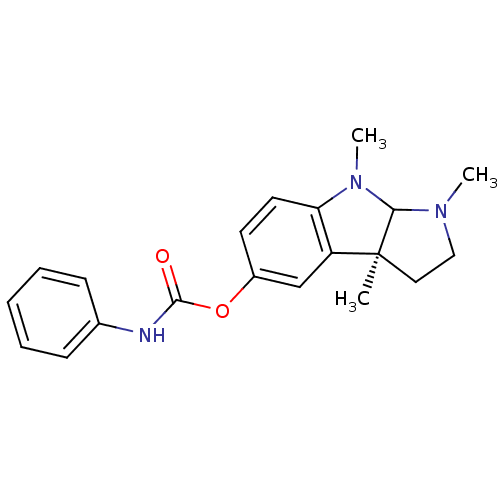
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shinada, M; Narumi, F; Osada, Y; Matsumoto, K; Yoshida, T; Higuchi, K; Kawasaki, T; Tanaka, H; Satoh, M Synthesis of phenserine analogues and evaluation of their cholinesterase inhibitory activities. Bioorg Med Chem20:4901-14 (2012) [PubMed] Article
Shinada, M; Narumi, F; Osada, Y; Matsumoto, K; Yoshida, T; Higuchi, K; Kawasaki, T; Tanaka, H; Satoh, M Synthesis of phenserine analogues and evaluation of their cholinesterase inhibitory activities. Bioorg Med Chem20:4901-14 (2012) [PubMed] Article