| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, inducible |
|---|
| Ligand | BDBM50148162 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_854481 (CHEMBL2155040) |
|---|
| IC50 | 350±n/a nM |
|---|
| Citation |  Stefani, HA; Gueogjan, K; Manarin, F; Farsky, SH; Zukerman-Schpector, J; Caracelli, I; Pizano Rodrigues, SR; Muscará, MN; Teixeira, SA; Santin, JR; Machado, ID; Bolonheis, SM; Curi, R; Vinolo, MA Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: effect on inducible nitric oxide synthase. Eur J Med Chem58:117-27 (2012) [PubMed] Article Stefani, HA; Gueogjan, K; Manarin, F; Farsky, SH; Zukerman-Schpector, J; Caracelli, I; Pizano Rodrigues, SR; Muscará, MN; Teixeira, SA; Santin, JR; Machado, ID; Bolonheis, SM; Curi, R; Vinolo, MA Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: effect on inducible nitric oxide synthase. Eur J Med Chem58:117-27 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, inducible |
|---|
| Name: | Nitric oxide synthase, inducible |
|---|
| Synonyms: | HEP-NOS | Hepatocyte NOS | Inducible NO synthase | Inducible NOS | NOS type II | NOS2 | NOS2A | NOS2_HUMAN | Nitric oxide synthase, inducible (iNOS) | iNOS |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 131141.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35228 |
|---|
| Residue: | 1153 |
|---|
| Sequence: | MACPWKFLFKTKFHQYAMNGEKDINNNVEKAPCATSSPVTQDDLQYHNLSKQQNESPQPL
VETGKKSPESLVKLDATPLSSPRHVRIKNWGSGMTFQDTLHHKAKGILTCRSKSCLGSIM
TPKSLTRGPRDKPTPPDELLPQAIEFVNQYYGSFKEAKIEEHLARVEAVTKEIETTGTYQ
LTGDELIFATKQAWRNAPRCIGRIQWSNLQVFDARSCSTAREMFEHICRHVRYSTNNGNI
RSAITVFPQRSDGKHDFRVWNAQLIRYAGYQMPDGSIRGDPANVEFTQLCIDLGWKPKYG
RFDVVPLVLQANGRDPELFEIPPDLVLEVAMEHPKYEWFRELELKWYALPAVANMLLEVG
GLEFPGCPFNGWYMGTEIGVRDFCDVQRYNILEEVGRRMGLETHKLASLWKDQAVVEINI
AVLHSFQKQNVTIMDHHSAAESFMKYMQNEYRSRGGCPADWIWLVPPMSGSITPVFHQEM
LNYVLSPFYYYQVEAWKTHVWQDEKRRPKRREIPLKVLVKAVLFACMLMRKTMASRVRVT
ILFATETGKSEALAWDLGALFSCAFNPKVVCMDKYRLSCLEEERLLLVVTSTFGNGDCPG
NGEKLKKSLFMLKELNNKFRYAVFGLGSSMYPRFCAFAHDIDQKLSHLGASQLTPMGEGD
ELSGQEDAFRSWAVQTFKAACETFDVRGKQHIQIPKLYTSNVTWDPHHYRLVQDSQPLDL
SKALSSMHAKNVFTMRLKSRQNLQSPTSSRATILVELSCEDGQGLNYLPGEHLGVCPGNQ
PALVQGILERVVDGPTPHQTVRLEALDESGSYWVSDKRLPPCSLSQALTYFLDITTPPTQ
LLLQKLAQVATEEPERQRLEALCQPSEYSKWKFTNSPTFLEVLEEFPSLRVSAGFLLSQL
PILKPRFYSISSSRDHTPTEIHLTVAVVTYHTRDGQGPLHHGVCSTWLNSLKPQDPVPCF
VRNASGFHLPEDPSHPCILIGPGTGIAPFRSFWQQRLHDSQHKGVRGGRMTLVFGCRRPD
EDHIYQEEMLEMAQKGVLHAVHTAYSRLPGKPKVYVQDILRQQLASEVLRVLHKEPGHLY
VCGDVRMARDVAHTLKQLVAAKLKLNEEQVEDYFFQLKSQKRYHEDIFGAVFPYEAKKDR
VAVQPSSLEMSAL
|
|
|
|---|
| BDBM50148162 |
|---|
| n/a |
|---|
| Name | BDBM50148162 |
|---|
| Synonyms: | 4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carboxylic acid ethyl ester | CHEMBL420671 | ETHYL 4-[(4-METHYLPYRIDIN-2-YL)AMINO]PIPERIDINE-1-CARBOXYLATE | Ethyl 4-[(4-methylpyridin-2-yl)amino]piperidine-1-carboxylate, 9 | ethyl 4-(4-methylpyridin-2-ylamino)piperidine-1-carboxylate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H21N3O2 |
|---|
| Mol. Mass. | 263.3354 |
|---|
| SMILES | CCOC(=O)N1CCC(CC1)Nc1cc(C)ccn1 |
|---|
| Structure | 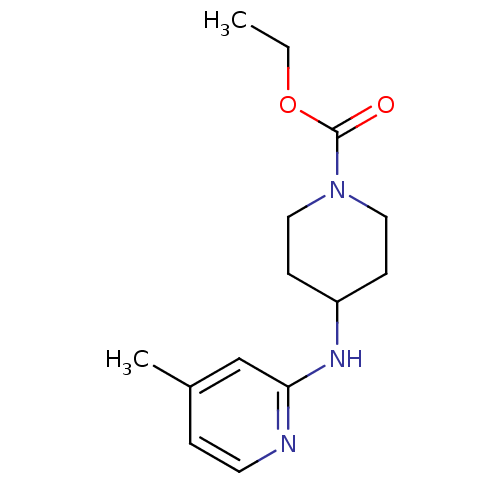
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Stefani, HA; Gueogjan, K; Manarin, F; Farsky, SH; Zukerman-Schpector, J; Caracelli, I; Pizano Rodrigues, SR; Muscará, MN; Teixeira, SA; Santin, JR; Machado, ID; Bolonheis, SM; Curi, R; Vinolo, MA Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: effect on inducible nitric oxide synthase. Eur J Med Chem58:117-27 (2012) [PubMed] Article
Stefani, HA; Gueogjan, K; Manarin, F; Farsky, SH; Zukerman-Schpector, J; Caracelli, I; Pizano Rodrigues, SR; Muscará, MN; Teixeira, SA; Santin, JR; Machado, ID; Bolonheis, SM; Curi, R; Vinolo, MA Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: effect on inducible nitric oxide synthase. Eur J Med Chem58:117-27 (2012) [PubMed] Article