| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50019260 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_50035 (CHEMBL666755) |
|---|
| IC50 | 2.09±n/a nM |
|---|
| Citation |  Tokarski, JS; Hopfinger, AJ Three-dimensional molecular shape analysis-quantitative structure-activity relationship of a series of cholecystokinin-A receptor antagonists. J Med Chem37:3639-54 (1994) [PubMed] Tokarski, JS; Hopfinger, AJ Three-dimensional molecular shape analysis-quantitative structure-activity relationship of a series of cholecystokinin-A receptor antagonists. J Med Chem37:3639-54 (1994) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49676.37 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin central 0 RAT::P30551 |
|---|
| Residue: | 444 |
|---|
| Sequence: | MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQI
LLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLK
DFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAAT
WCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVM
VVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQL
SSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAE
KHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEE
DGRTIRALLSRYSYSHMSTSAPPP
|
|
|
|---|
| BDBM50019260 |
|---|
| n/a |
|---|
| Name | BDBM50019260 |
|---|
| Synonyms: | 1H-Indole-2-carboxylic acid [5-(2-fluoro-phenyl)-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl]-amide | 1H-Indole-2-carboxylic acid [5-(2-fluoro-phenyl)-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl]-amide: 0.16CH2Cl2 | CCK antagonist synthetic 21 | CHEMBL306806 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H17FN4O2 |
|---|
| Mol. Mass. | 412.4158 |
|---|
| SMILES | Fc1ccccc1C1=NC(NC(=O)c2cc3ccccc3[nH]2)C(=O)Nc2ccccc12 |t:8| |
|---|
| Structure | 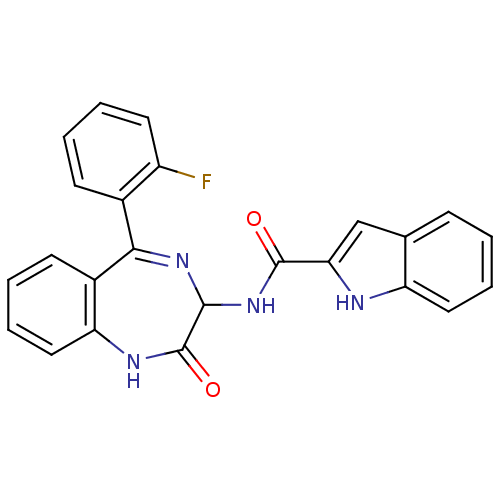
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tokarski, JS; Hopfinger, AJ Three-dimensional molecular shape analysis-quantitative structure-activity relationship of a series of cholecystokinin-A receptor antagonists. J Med Chem37:3639-54 (1994) [PubMed]
Tokarski, JS; Hopfinger, AJ Three-dimensional molecular shape analysis-quantitative structure-activity relationship of a series of cholecystokinin-A receptor antagonists. J Med Chem37:3639-54 (1994) [PubMed]