 Found 418 hits with Last Name = 'hopfinger' and Initial = 'aj'
Found 418 hits with Last Name = 'hopfinger' and Initial = 'aj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147896
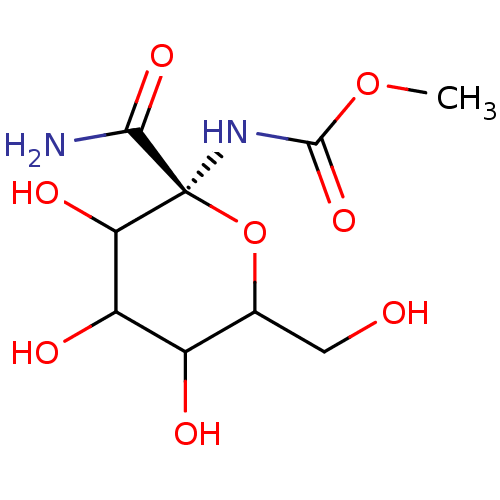
(((S)-2-Carbamoyl-3,4,5-trihydroxy-6-hydroxymethyl-...)Show InChI InChI=1S/C9H16N2O8/c1-18-8(17)11-9(7(10)16)6(15)5(14)4(13)3(2-12)19-9/h3-6,12-15H,2H2,1H3,(H2,10,16)(H,11,17)/t3?,4?,5?,6?,9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50240802
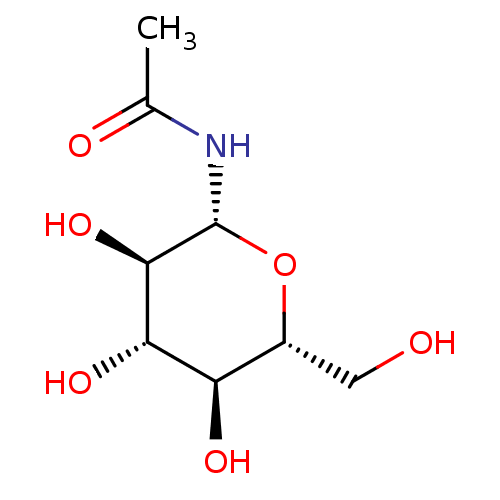
(1-N-ACETYL-BETA-D-GLUCOSAMINE | CHEMBL335315 | N-(...)Show SMILES CC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C8H15NO6/c1-3(11)9-8-7(14)6(13)5(12)4(2-10)15-8/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6+,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422220
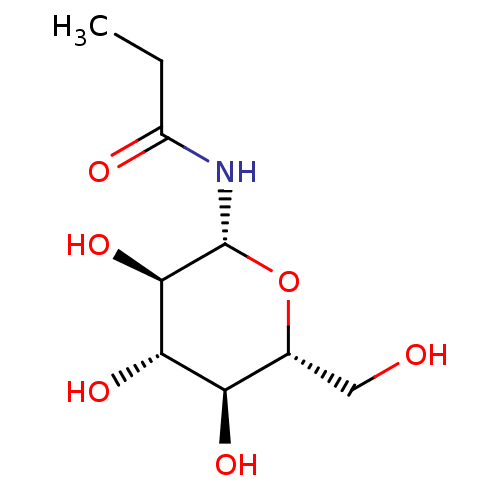
(CHEMBL334587)Show SMILES CCC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C9H17NO6/c1-2-5(12)10-9-8(15)7(14)6(13)4(3-11)16-9/h4,6-9,11,13-15H,2-3H2,1H3,(H,10,12)/t4-,6-,7+,8-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147901
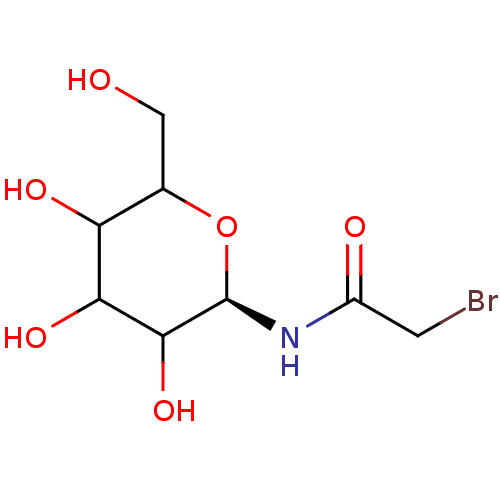
(2-Bromo-N-((R)-3,4,5-trihydroxy-6-hydroxymethyl-te...)Show InChI InChI=1S/C8H14BrNO6/c9-1-4(12)10-8-7(15)6(14)5(13)3(2-11)16-8/h3,5-8,11,13-15H,1-2H2,(H,10,12)/t3?,5?,6?,7?,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422216
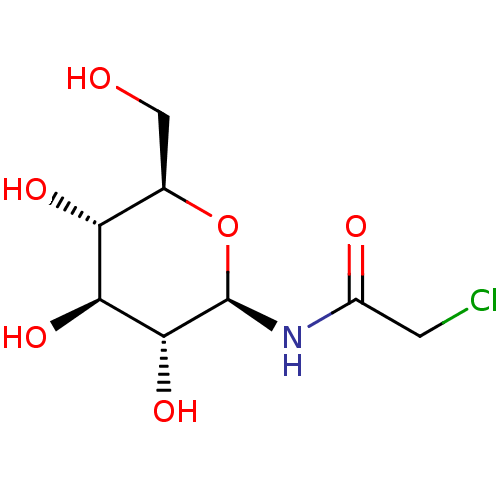
(CHEMBL336310)Show SMILES OC[C@H]1O[C@@H](NC(=O)CCl)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H14ClNO6/c9-1-4(12)10-8-7(15)6(14)5(13)3(2-11)16-8/h3,5-8,11,13-15H,1-2H2,(H,10,12)/t3-,5-,6+,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50240801
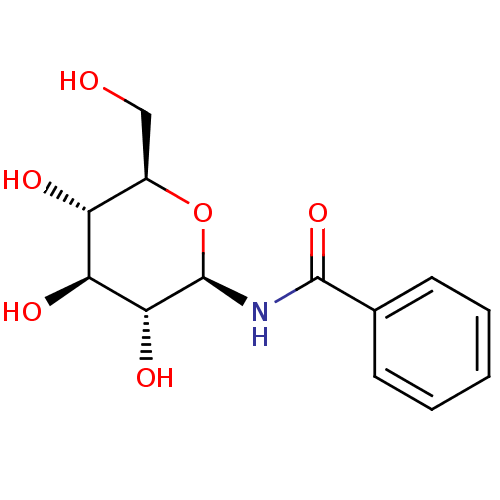
(CHEMBL131967 | N-((2R,3R,4S,5S,6R)-3,4,5-trihydrox...)Show SMILES OC[C@H]1O[C@@H](NC(=O)c2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H17NO6/c15-6-8-9(16)10(17)11(18)13(20-8)14-12(19)7-4-2-1-3-5-7/h1-5,8-11,13,15-18H,6H2,(H,14,19)/t8-,9-,10+,11-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422223
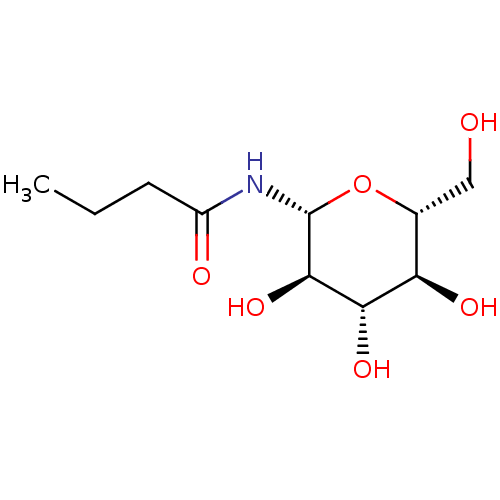
(CHEMBL423714)Show SMILES CCCC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C10H19NO6/c1-2-3-6(13)11-10-9(16)8(15)7(14)5(4-12)17-10/h5,7-10,12,14-16H,2-4H2,1H3,(H,11,13)/t5-,7-,8+,9-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50363870

(CHEMBL132020)Show SMILES NC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C7H14N2O6/c8-7(14)9-6-5(13)4(12)3(11)2(1-10)15-6/h2-6,10-13H,1H2,(H3,8,9,14)/t2-,3-,4+,5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147917
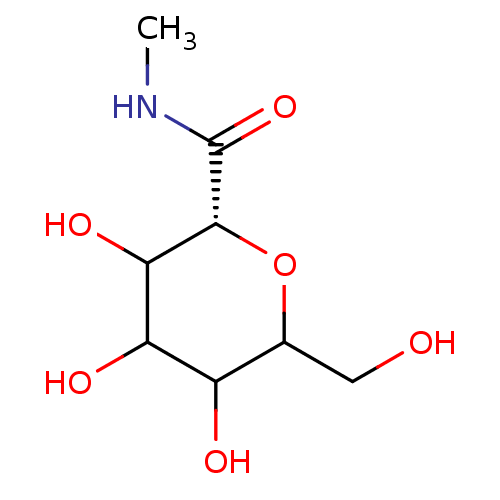
((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C8H15NO6/c1-9-8(14)7-6(13)5(12)4(11)3(2-10)15-7/h3-7,10-13H,2H2,1H3,(H,9,14)/t3?,4?,5?,6?,7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147928
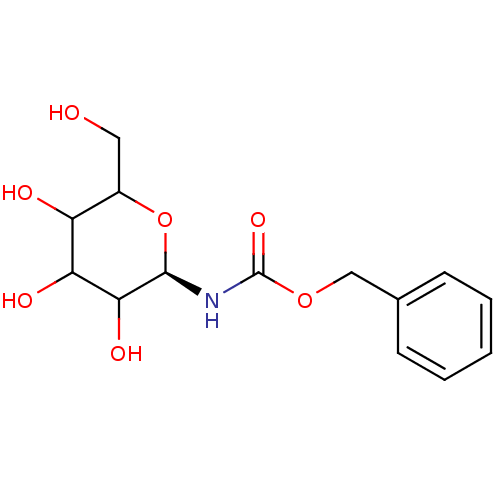
(((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-p...)Show InChI InChI=1S/C14H19NO7/c16-6-9-10(17)11(18)12(19)13(22-9)15-14(20)21-7-8-4-2-1-3-5-8/h1-5,9-13,16-19H,6-7H2,(H,15,20)/t9?,10?,11?,12?,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147912
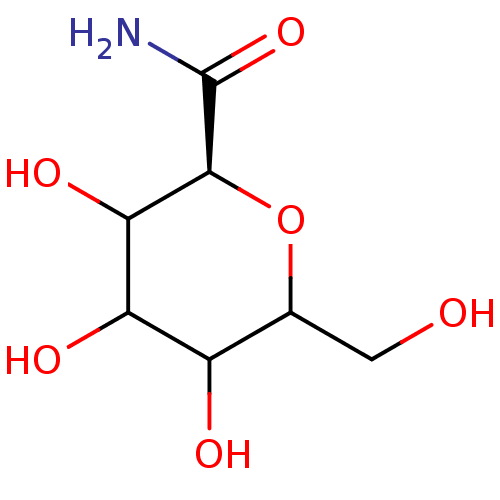
((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H13NO6/c8-7(13)6-5(12)4(11)3(10)2(1-9)14-6/h2-6,9-12H,1H2,(H2,8,13)/t2?,3?,4?,5?,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147924

(((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-p...)Show InChI InChI=1S/C8H16N2O6/c9-1-4(12)10-8-7(15)6(14)5(13)3(2-11)16-8/h3,5-8,11,13-15H,1-2,9H2,(H,10,12)/p+1/t3?,5?,6?,7?,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147898
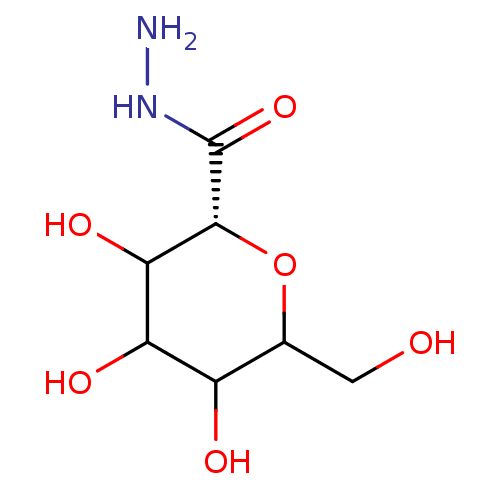
((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H14N2O6/c8-9-7(14)6-5(13)4(12)3(11)2(1-10)15-6/h2-6,10-13H,1,8H2,(H,9,14)/t2?,3?,4?,5?,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147890

((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H13NO6/c8-7(13)6-5(12)4(11)3(10)2(1-9)14-6/h2-6,9-12H,1H2,(H2,8,13)/t2?,3?,4?,5?,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422219
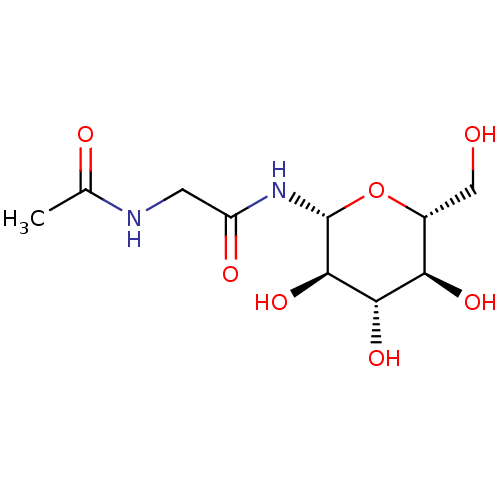
(CHEMBL133222)Show SMILES CC(=O)NCC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C10H18N2O7/c1-4(14)11-2-6(15)12-10-9(18)8(17)7(16)5(3-13)19-10/h5,7-10,13,16-18H,2-3H2,1H3,(H,11,14)(H,12,15)/t5-,7-,8+,9-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147921

((S)-2-Hydroxymethyl-6-mercapto-tetrahydro-pyran-3,...)Show InChI InChI=1S/C6H12O5S/c7-1-2-3(8)4(9)5(10)6(12)11-2/h2-10,12H,1H2/t2?,3?,4?,5?,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147905

((R)-2,6-Bis-hydroxymethyl-tetrahydro-pyran-3,4,5-t...)Show InChI InChI=1S/C7H14O6/c8-1-3-5(10)7(12)6(11)4(2-9)13-3/h3-12H,1-2H2/t3-,4?,5?,6?,7?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147883

((S)-6-Hydroxymethyl-tetrahydro-pyran-2,3,4,5-tetra...)Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2?,3?,4?,5?,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422212
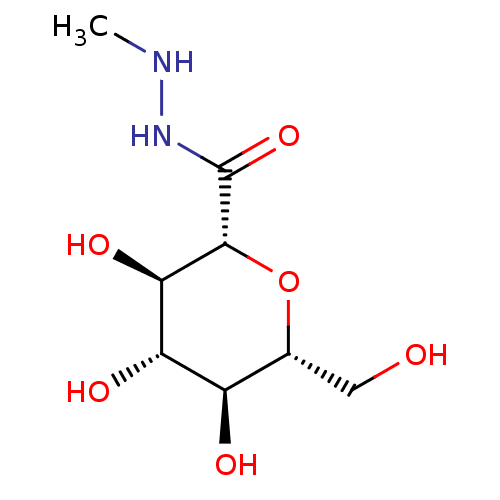
(CHEMBL132462)Show SMILES CNNC(=O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C8H16N2O6/c1-9-10-8(15)7-6(14)5(13)4(12)3(2-11)16-7/h3-7,9,11-14H,2H2,1H3,(H,10,15)/t3-,4-,5+,6-,7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422213

(CHEMBL134001)Show SMILES OC[C@H]1S[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H12O5S/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147913
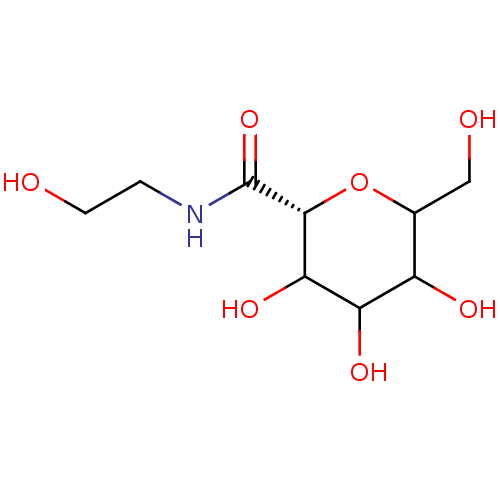
((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C9H17NO7/c11-2-1-10-9(16)8-7(15)6(14)5(13)4(3-12)17-8/h4-8,11-15H,1-3H2,(H,10,16)/t4?,5?,6?,7?,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147918

((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C8H14O7/c1-14-8(13)7-6(12)5(11)4(10)3(2-9)15-7/h3-7,9-12H,2H2,1H3/t3?,4?,5?,6?,7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147915

((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H14N2O6/c8-9-7(14)6-5(13)4(12)3(11)2(1-10)15-6/h2-6,10-13H,1,8H2,(H,9,14)/t2?,3?,4?,5?,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422221
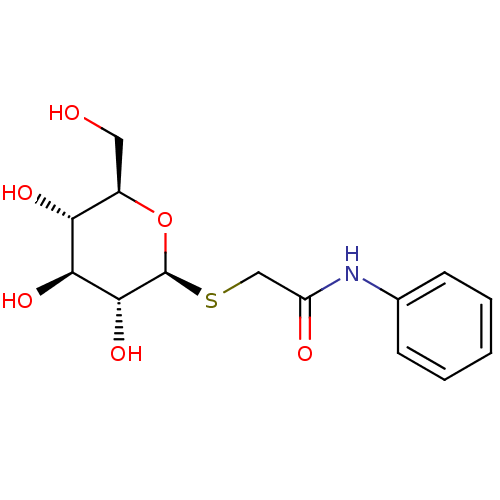
(CHEMBL133751)Show SMILES OC[C@H]1O[C@@H](SCC(=O)Nc2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C14H19NO6S/c16-6-9-11(18)12(19)13(20)14(21-9)22-7-10(17)15-8-4-2-1-3-5-8/h1-5,9,11-14,16,18-20H,6-7H2,(H,15,17)/t9-,11-,12+,13-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147920
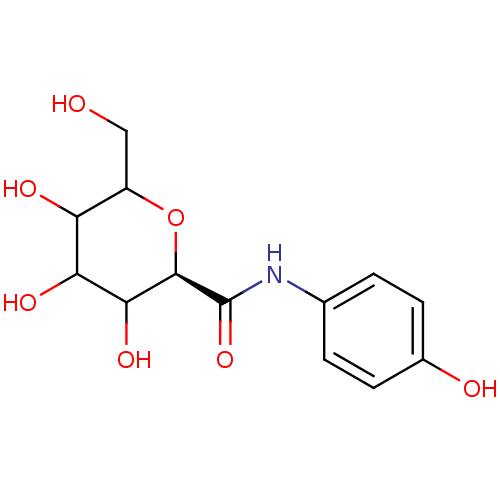
((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C13H17NO7/c15-5-8-9(17)10(18)11(19)12(21-8)13(20)14-6-1-3-7(16)4-2-6/h1-4,8-12,15-19H,5H2,(H,14,20)/t8?,9?,10?,11?,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147902
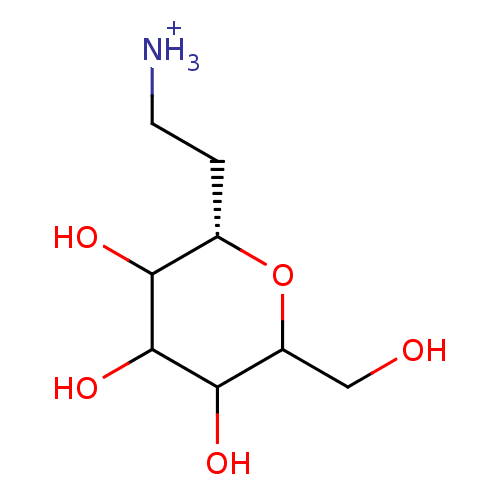
(2-((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro...)Show InChI InChI=1S/C8H17NO5/c9-2-1-4-6(11)8(13)7(12)5(3-10)14-4/h4-8,10-13H,1-3,9H2/p+1/t4-,5?,6?,7?,8?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147889
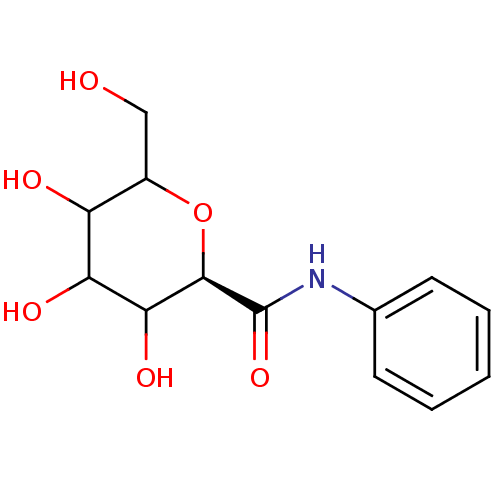
((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C13H17NO6/c15-6-8-9(16)10(17)11(18)12(20-8)13(19)14-7-4-2-1-3-5-7/h1-5,8-12,15-18H,6H2,(H,14,19)/t8?,9?,10?,11?,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147887
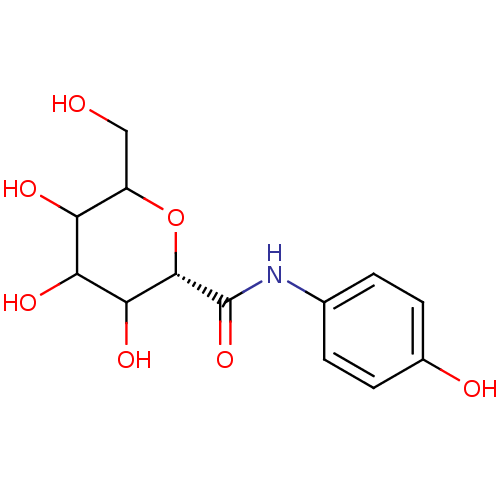
((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C13H17NO7/c15-5-8-9(17)10(18)11(19)12(21-8)13(20)14-6-1-3-7(16)4-2-6/h1-4,8-12,15-19H,5H2,(H,14,20)/t8?,9?,10?,11?,12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147910
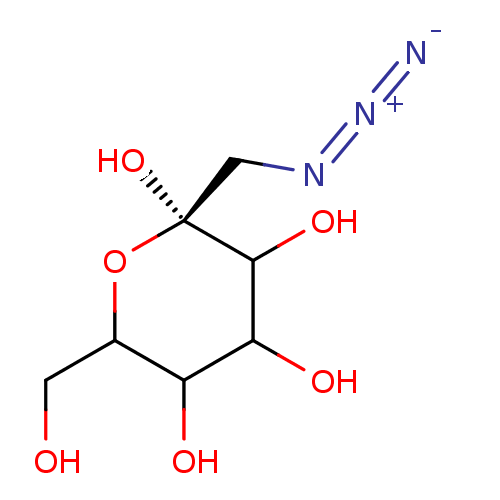
((S)-2-Azidomethyl-6-hydroxymethyl-tetrahydro-pyran...)Show InChI InChI=1S/C7H13N3O6/c8-10-9-2-7(15)6(14)5(13)4(12)3(1-11)16-7/h3-6,11-15H,1-2H2/t3?,4?,5?,6?,7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM34103
(D-glucose | dextrose | glucose)Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147916
(((S)-2,3,4,5-Tetrahydroxy-6-hydroxymethyl-tetrahyd...)Show InChI InChI=1S/C8H13NO6/c9-2-1-8(14)7(13)6(12)5(11)4(3-10)15-8/h4-7,10-14H,1,3H2/t4?,5?,6?,7?,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147906

((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C9H14F3NO6/c10-9(11,12)2-13-8(18)7-6(17)5(16)4(15)3(1-14)19-7/h3-7,14-17H,1-2H2,(H,13,18)/t3?,4?,5?,6?,7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147923
(((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-p...)Show InChI InChI=1S/C8H13NO5/c9-2-1-4-6(11)8(13)7(12)5(3-10)14-4/h4-8,10-13H,1,3H2/t4-,5?,6?,7?,8?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147909

((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C13H17NO6/c15-6-8-9(16)10(17)11(18)12(20-8)13(19)14-7-4-2-1-3-5-7/h1-5,8-12,15-18H,6H2,(H,14,19)/t8?,9?,10?,11?,12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147897
((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H12O7/c8-1-2-3(9)4(10)5(11)6(14-2)7(12)13/h2-6,8-11H,1H2,(H,12,13)/p-1/t2?,3?,4?,5?,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147907

((S)-2,6-Bis-hydroxymethyl-tetrahydro-pyran-2,3,4,5...)Show InChI InChI=1S/C7H14O7/c8-1-3-4(10)5(11)6(12)7(13,2-9)14-3/h3-6,8-13H,1-2H2/t3?,4?,5?,6?,7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147894

((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H15NO5/c8-1-3-5(10)7(12)6(11)4(2-9)13-3/h3-7,9-12H,1-2,8H2/p+1/t3-,4?,5?,6?,7?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.68E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147908

((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C9H17NO7/c11-2-1-10-9(16)8-7(15)6(14)5(13)4(3-12)17-8/h4-8,11-15H,1-3H2,(H,10,16)/t4?,5?,6?,7?,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.69E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422218
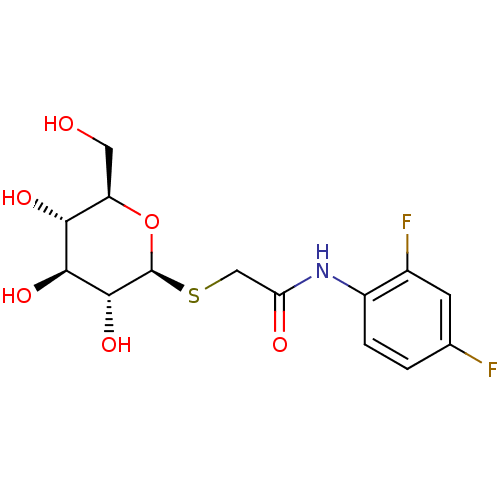
(CHEMBL130754)Show SMILES OC[C@H]1O[C@@H](SCC(=O)Nc2ccc(F)cc2F)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C14H17F2NO6S/c15-6-1-2-8(7(16)3-6)17-10(19)5-24-14-13(22)12(21)11(20)9(4-18)23-14/h1-3,9,11-14,18,20-22H,4-5H2,(H,17,19)/t9-,11-,12+,13-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.89E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50422215
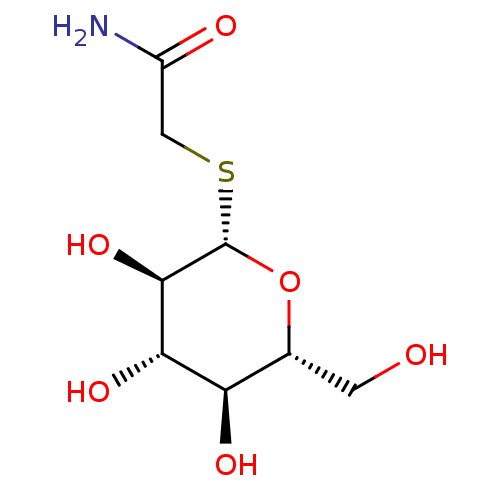
(CHEMBL134228)Show SMILES NC(=O)CS[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C8H15NO6S/c9-4(11)2-16-8-7(14)6(13)5(12)3(1-10)15-8/h3,5-8,10,12-14H,1-2H2,(H2,9,11)/t3-,5-,6+,7-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147904

((R)-2-Azidomethyl-6-hydroxymethyl-tetrahydro-pyran...)Show InChI InChI=1S/C7H13N3O5/c8-10-9-1-3-5(12)7(14)6(13)4(2-11)15-3/h3-7,11-14H,1-2H2/t3-,4?,5?,6?,7?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.24E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147925
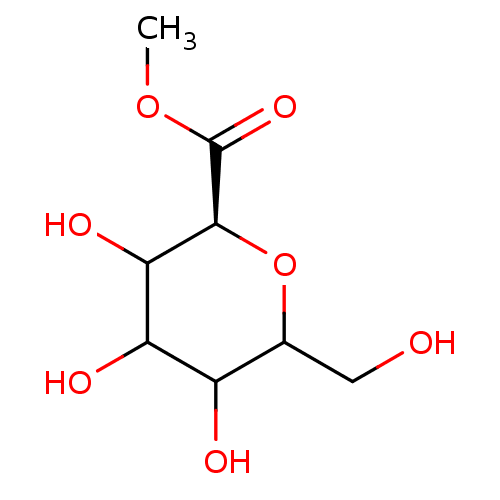
((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C8H14O7/c1-14-8(13)7-6(12)5(11)4(10)3(2-9)15-7/h3-7,9-12H,2H2,1H3/t3?,4?,5?,6?,7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.42E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147927
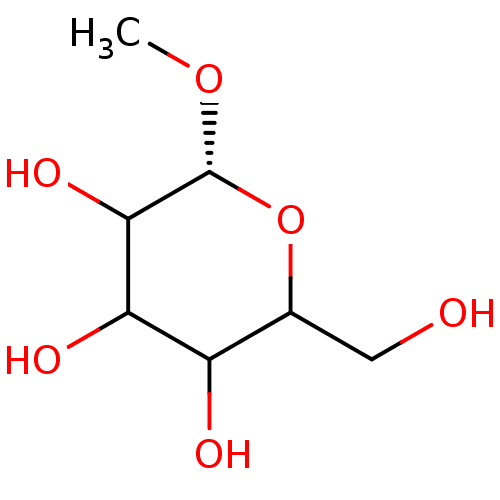
((R)-2-Hydroxymethyl-6-methoxy-tetrahydro-pyran-3,4...)Show InChI InChI=1S/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3/t3?,4?,5?,6?,7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.47E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147886
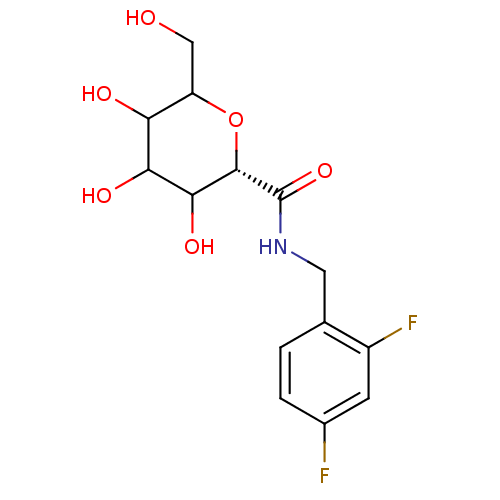
((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show SMILES OCC1O[C@@H](C(O)C(O)C1O)C(=O)NCc1ccc(F)cc1F Show InChI InChI=1S/C14H17F2NO6/c15-7-2-1-6(8(16)3-7)4-17-14(22)13-12(21)11(20)10(19)9(5-18)23-13/h1-3,9-13,18-21H,4-5H2,(H,17,22)/t9?,10?,11?,12?,13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.72E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147884
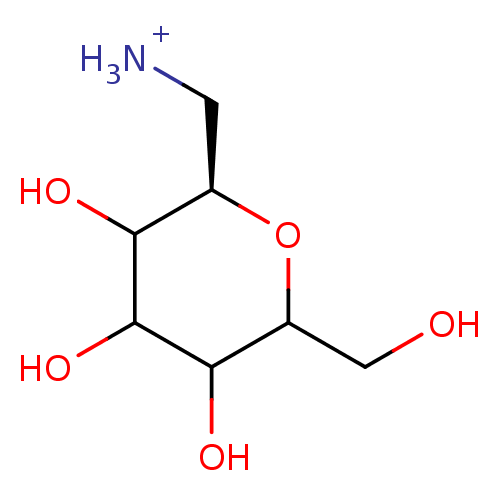
((R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H15NO5/c8-1-3-5(10)7(12)6(11)4(2-9)13-3/h3-7,9-12H,1-2,8H2/p+1/t3-,4?,5?,6?,7?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.45E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147900
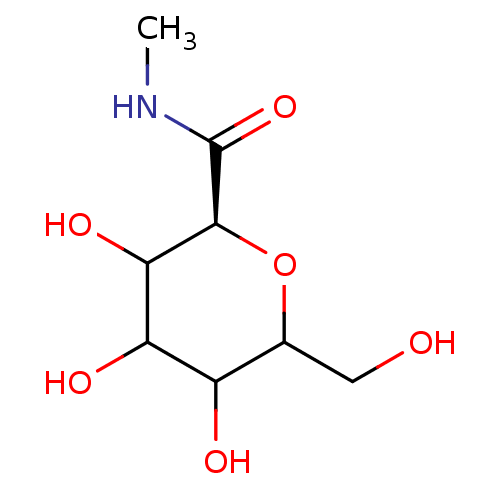
((S)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C8H15NO6/c1-9-8(14)7-6(13)5(12)4(11)3(2-10)15-7/h3-7,10-13H,2H2,1H3,(H,9,14)/t3?,4?,5?,6?,7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.67E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50147926
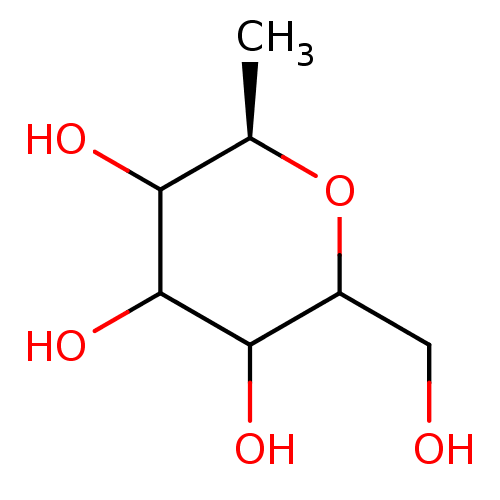
((R)-2-Hydroxymethyl-6-methyl-tetrahydro-pyran-3,4,...)Show InChI InChI=1S/C7H14O5/c1-3-5(9)7(11)6(10)4(2-8)12-3/h3-11H,2H2,1H3/t3-,4?,5?,6?,7?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.31E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory binding constant against glycogen phosphorylase B |
J Med Chem 47: 3075-88 (2004)
Article DOI: 10.1021/jm030586a
BindingDB Entry DOI: 10.7270/Q2930TXG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Binding affinity against cholecystokinin type A receptor of rat pancreas |
J Med Chem 37: 3639-54 (1994)
BindingDB Entry DOI: 10.7270/Q2SB46ZR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against acetylcholinesterase |
J Med Chem 35: 584-9 (1992)
BindingDB Entry DOI: 10.7270/Q20G3J3J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50367639

(CHEMBL1907855)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1F |r,c:9| Show InChI InChI=1S/C25H19FN4O2/c1-30-21-13-7-4-10-17(21)22(16-9-3-5-11-18(16)26)28-23(25(30)32)29-24(31)20-14-15-8-2-6-12-19(15)27-20/h2-14,23,27H,1H3,(H,29,31)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Binding affinity against cholecystokinin type A receptor of rat pancreas |
J Med Chem 37: 3639-54 (1994)
BindingDB Entry DOI: 10.7270/Q2SB46ZR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data