| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histidine-rich protein PFHRP-II |
|---|
| Ligand | BDBM22985 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_328025 (CHEMBL863915) |
|---|
| IC50 | 60000±n/a nM |
|---|
| Citation |  Melnyk, P; Leroux, V; Sergheraert, C; Grellier, P Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg Med Chem Lett16:31-5 (2005) [PubMed] Article Melnyk, P; Leroux, V; Sergheraert, C; Grellier, P Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg Med Chem Lett16:31-5 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histidine-rich protein PFHRP-II |
|---|
| Name: | Histidine-rich protein PFHRP-II |
|---|
| Synonyms: | HRP1_PLAFA | Histidine-rich protein | Histidine-rich protein PFHRP-II |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 35156.49 |
|---|
| Organism: | Plasmodium falciparum |
|---|
| Description: | ChEMBL_500704 |
|---|
| Residue: | 332 |
|---|
| Sequence: | MVSFSKNKVLSAAVFASVLLLDNNNSAFNNNLCSKNAKGLNLNKRLLHETQAHVDDAHHA
HHVADAHHAHHAHHAADAHHAHHAADAHHAHHAADAHHAHHAADAHHAHHAADAHHAHHA
ADAHHAHHAADAHHAHHAADAHHAHHAADAHHAHHAAYAHHAHHASDAHHAADAHHAAYA
HHAHHAADAHHAADAHHAAYAHHAHHAADAHHAADAHHATDAHHAHHAADAHHATDAHHA
ADAHHAADAHHATDAHHAADAHHATDAHHAADAHHAADAHHATDSHHAHHAADAHHAAAH
HATDAHHAAAHHATDAHHAAAHHEAATHCLRH
|
|
|
|---|
| BDBM22985 |
|---|
| n/a |
|---|
| Name | BDBM22985 |
|---|
| Synonyms: | Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chlorochin | Chloroquine | Chloroquine, 17 | med.21724, Compound 8 | {4-[(7-chloroquinolin-4-yl)amino]pentyl}diethylamine |
|---|
| Type | Antimalarial |
|---|
| Emp. Form. | C18H26ClN3 |
|---|
| Mol. Mass. | 319.872 |
|---|
| SMILES | CCN(CC)CCCC(C)Nc1ccnc2cc(Cl)ccc12 |
|---|
| Structure | 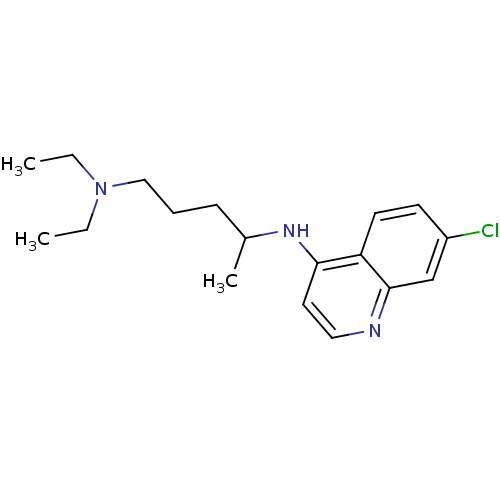
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Melnyk, P; Leroux, V; Sergheraert, C; Grellier, P Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg Med Chem Lett16:31-5 (2005) [PubMed] Article
Melnyk, P; Leroux, V; Sergheraert, C; Grellier, P Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg Med Chem Lett16:31-5 (2005) [PubMed] Article