

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635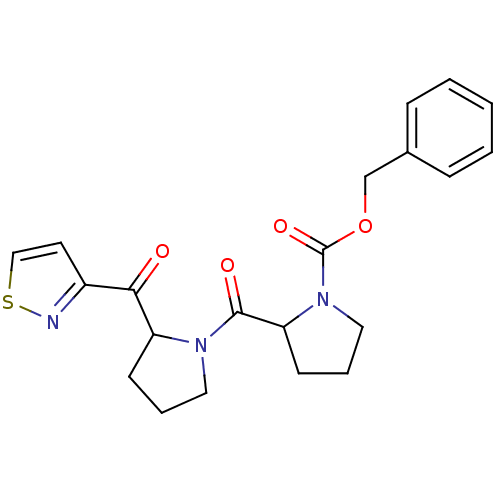 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135637 (1-{(S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135634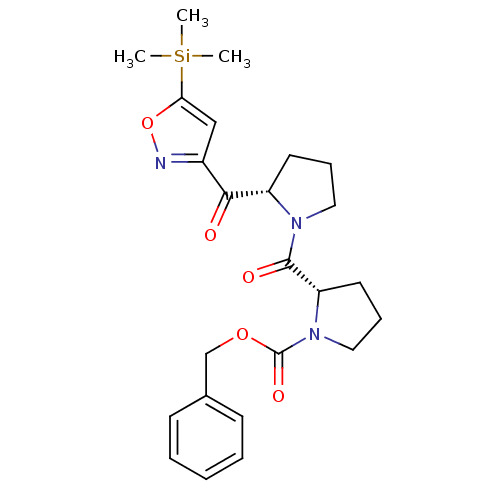 ((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of PKD2 ( assessed as residual activity at 1 uM ) by TR-FRET assay | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135636 ((S)-2-[(S)-2-(5-Cyano-isoxazole-3-carbonyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human; Moderately active | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135634 ((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135632 ((S)-2-[(S)-2-(Isoxazole-3-carbonyl)-pyrrolidine-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135636 ((S)-2-[(S)-2-(5-Cyano-isoxazole-3-carbonyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135632 ((S)-2-[(S)-2-(Isoxazole-3-carbonyl)-pyrrolidine-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50481616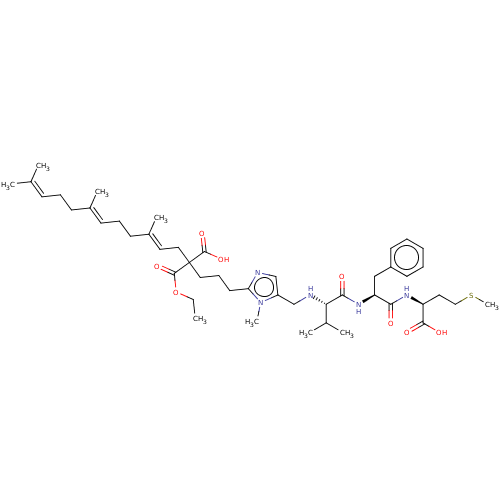 (CHEMBL590127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette Curated by ChEMBL | Assay Description Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis | Bioorg Med Chem 18: 543-56 (2010) Article DOI: 10.1016/j.bmc.2009.12.017 BindingDB Entry DOI: 10.7270/Q2VM4G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50481625 (CHEMBL599795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette Curated by ChEMBL | Assay Description Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis | Bioorg Med Chem 18: 543-56 (2010) Article DOI: 10.1016/j.bmc.2009.12.017 BindingDB Entry DOI: 10.7270/Q2VM4G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50481615 (CHEMBL590126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette Curated by ChEMBL | Assay Description Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis | Bioorg Med Chem 18: 543-56 (2010) Article DOI: 10.1016/j.bmc.2009.12.017 BindingDB Entry DOI: 10.7270/Q2VM4G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135631 (3-[(S)-1-((S)-1-Benzyloxycarbonyl-pyrrolidine-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi; Moderately active | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135631 (3-[(S)-1-((S)-1-Benzyloxycarbonyl-pyrrolidine-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50481616 (CHEMBL590127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette Curated by ChEMBL | Assay Description Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-substrate-inhibitor complex | Bioorg Med Chem 18: 543-56 (2010) Article DOI: 10.1016/j.bmc.2009.12.017 BindingDB Entry DOI: 10.7270/Q2VM4G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50481616 (CHEMBL590127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette Curated by ChEMBL | Assay Description Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-inhibitor complex | Bioorg Med Chem 18: 543-56 (2010) Article DOI: 10.1016/j.bmc.2009.12.017 BindingDB Entry DOI: 10.7270/Q2VM4G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50481615 (CHEMBL590126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette Curated by ChEMBL | Assay Description Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-substrate-inhibitor complex | Bioorg Med Chem 18: 543-56 (2010) Article DOI: 10.1016/j.bmc.2009.12.017 BindingDB Entry DOI: 10.7270/Q2VM4G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50481615 (CHEMBL590126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette Curated by ChEMBL | Assay Description Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-inhibitor complex | Bioorg Med Chem 18: 543-56 (2010) Article DOI: 10.1016/j.bmc.2009.12.017 BindingDB Entry DOI: 10.7270/Q2VM4G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Saccharomyces cerevisiae (Baker's yeast)) | BDBM50067584 ((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Centre de Recherche de Gif | Assay Description Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per... | J Enzyme Inhib Med Chem 28: 163-71 (2013) Article DOI: 10.3109/14756366.2011.643302 BindingDB Entry DOI: 10.7270/Q2319TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50123594 ((7-Chloro-quinolin-4-yl)-{3-[4-(3-dibutylamino-pro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075135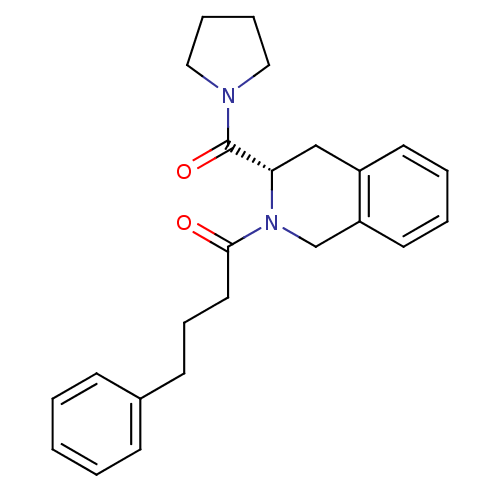 ((S)-4-phenyl-1-(3-(pyrrolidine-1-carbonyl)-3,4-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075132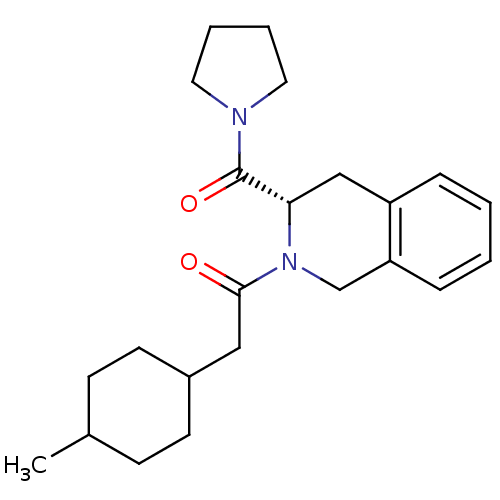 (2-(4-Methyl-cyclohexyl)-1-[(S)-3-(pyrrolidine-1-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50139469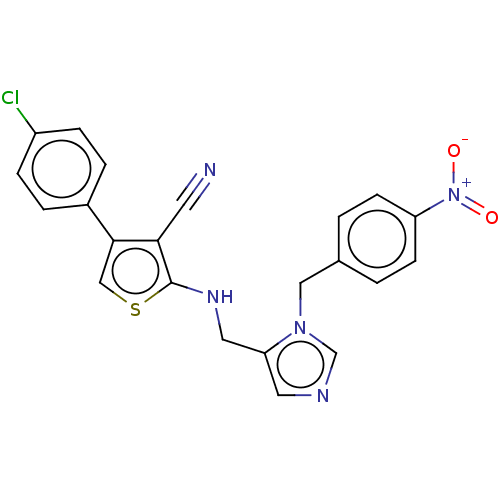 (CHEMBL3763335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301 Curated by ChEMBL | Assay Description Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay | Eur J Med Chem 109: 173-86 (2016) Article DOI: 10.1016/j.ejmech.2015.12.045 BindingDB Entry DOI: 10.7270/Q25B04C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase alpha subunit/subunit beta (Trypanosoma brucei brucei) | BDBM50067584 ((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif | Assay Description Assays were realized on 96-well plates, as described for human FTase with the dansylated peptide Dansyl-GCAIM and the solution contains 15 μL of... | J Enzyme Inhib Med Chem 28: 163-71 (2013) Article DOI: 10.3109/14756366.2011.643302 BindingDB Entry DOI: 10.7270/Q2319TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50139637 (CHEMBL3765675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301 Curated by ChEMBL | Assay Description Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay | Eur J Med Chem 109: 173-86 (2016) Article DOI: 10.1016/j.ejmech.2015.12.045 BindingDB Entry DOI: 10.7270/Q25B04C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075126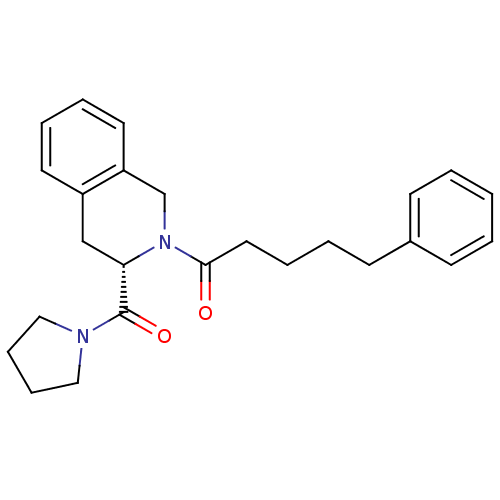 (5-Phenyl-1-[(S)-3-(pyrrolidine-1-carbonyl)-3,4-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50123579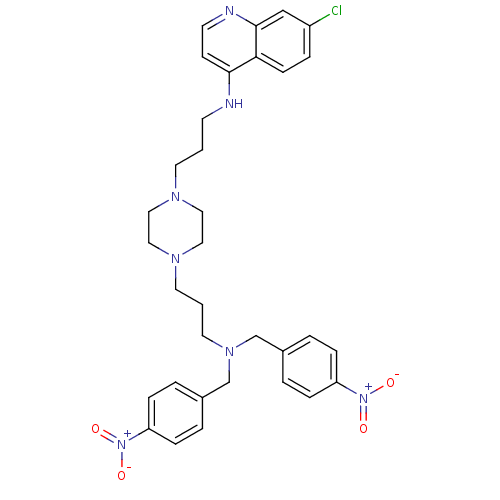 (CHEMBL345958 | [3-(4-{3-[Bis-(4-nitro-benzyl)-amin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051539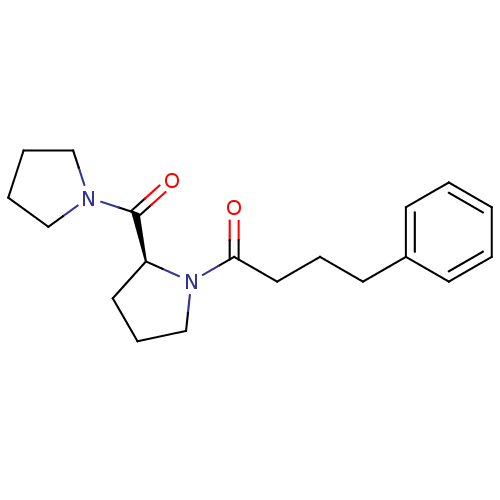 ((S)-4-phenyl-1-(2-(pyrrolidine-1-carbonyl)pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50067584 ((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif | Assay Description Assays were realized on 96-well plates, as described for yeast FTase but octyl-D-glucopyranoside (0.18%) was used instead of CHAPS and the solution c... | J Enzyme Inhib Med Chem 28: 163-71 (2013) Article DOI: 10.3109/14756366.2011.643302 BindingDB Entry DOI: 10.7270/Q2319TSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075134 (4-(4-Chloro-2-methyl-phenoxy)-1-[(S)-3-(pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075129 (1-[(S)-3-(Pyrrolidine-1-carbonyl)-3,4-dihydro-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075128 (CHEMBL138674 | {6-Oxo-6-[(S)-3-(pyrrolidine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075125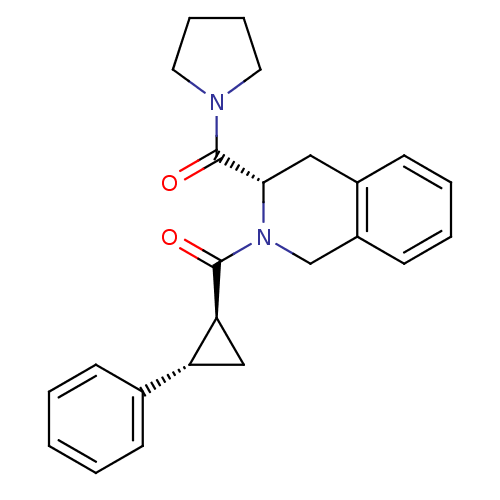 (((1S,2S)-2-Phenyl-cyclopropyl)-[(S)-3-(pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50123587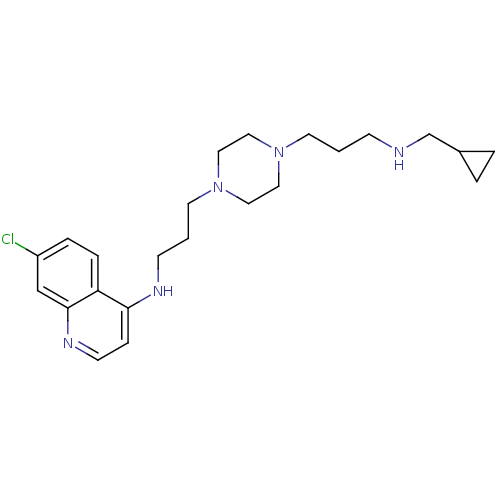 ((7-Chloro-quinolin-4-yl)-(3-{4-[3-(cyclopropylmeth...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50409777 (CHEMBL2111198) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075130 (1-[(S)-3-(Pyrrolidine-1-carbonyl)-3,4-dihydro-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50123575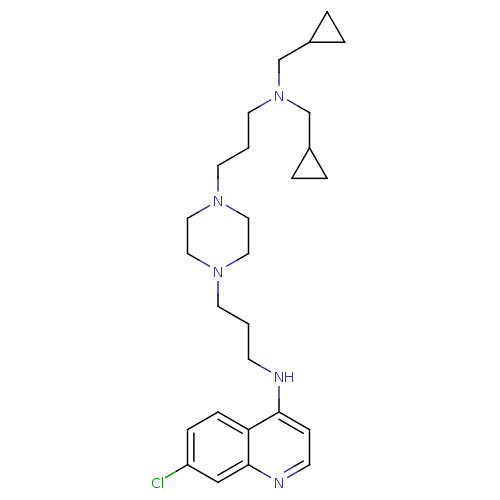 ((3-{4-[3-(Bis-cyclopropylmethyl-amino)-propyl]-pip...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131276 (CHEMBL328470 | N-(3-{4-[3-(7-Chloro-quinolin-4-yla...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2 Curated by ChEMBL | Assay Description Inhibitory activity against mammalian Aminopeptidase N (APN) | Bioorg Med Chem Lett 13: 2659-62 (2003) BindingDB Entry DOI: 10.7270/Q20G3KPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50123577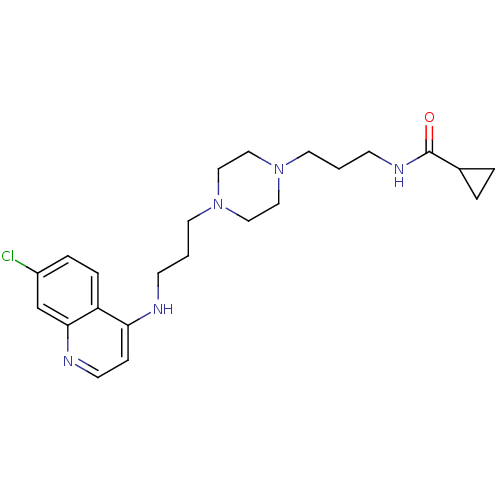 (CHEMBL152862 | Cyclopropanecarboxylic acid (3-{4-[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50123561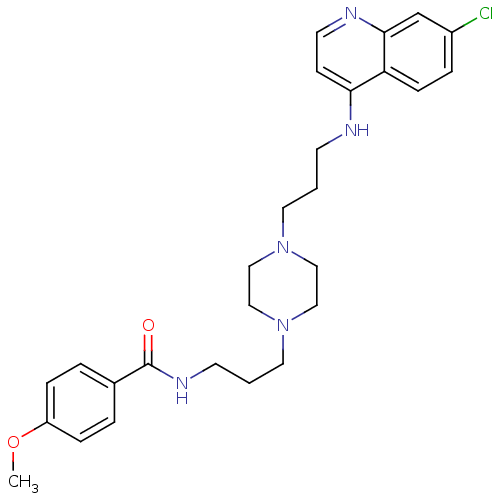 (CHEMBL149751 | N-(3-(4-(3-(7-chloroquinolin-4-ylam...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50123569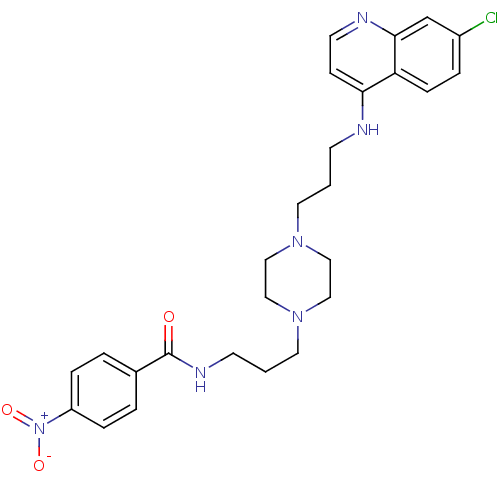 (CHEMBL357111 | N-(3-{4-[3-(7-Chloro-quinolin-4-yla...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille II Curated by ChEMBL | Assay Description In vitro inhibition of beta-hematin formation | J Med Chem 46: 542-57 (2003) Article DOI: 10.1021/jm020960r BindingDB Entry DOI: 10.7270/Q27W6DDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 219 total ) | Next | Last >> |