

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Acetylcholinesterase | ||
| Ligand | BDBM50135148 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEBML_29848 | ||
| IC50 | 6166±n/a nM | ||
| Citation |  Zaheer-ul-Haq, na; Wellenzohn, B; Tonmunphean, S; Khalid, A; Choudhary, MI; Rode, BM 3D-QSAR studies on natural acetylcholinesterase inhibitors of Sarcococca saligna by comparative molecular field analysis (CoMFA). Bioorg Med Chem Lett13:4375-80 (2003) [PubMed] Zaheer-ul-Haq, na; Wellenzohn, B; Tonmunphean, S; Khalid, A; Choudhary, MI; Rode, BM 3D-QSAR studies on natural acetylcholinesterase inhibitors of Sarcococca saligna by comparative molecular field analysis (CoMFA). Bioorg Med Chem Lett13:4375-80 (2003) [PubMed] | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Acetylcholinesterase | |||
| Name: | Acetylcholinesterase | ||
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 67792.70 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P22303 | ||
| Residue: | 614 | ||
| Sequence: |
| ||
| BDBM50135148 | |||
| n/a | |||
| Name | BDBM50135148 | ||
| Synonyms: | 1N-[14-(1-dimethylaminoethyl)-2,15-dimethyl-(1S,7S,10R,11S)-tetracyclo[8.7.0.02,7.011,15]heptadec-13-en-5-yl]-1N,3,4-trimethyl-(E)-2-pentenamide | 3,4-Dimethyl-pent-2-enoic acid [(S)-17-(1-dimethylamino-ethyl)-10,13-dimethyl-2,3,4,5,6,7,8,9,10,11,12,13,14,15-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl]-methyl-amide | CHEMBL424100 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C31H52N2O | ||
| Mol. Mass. | 468.7574 | ||
| SMILES | CC(C)C(\C)=C\C(=O)N(C)[C@H]1CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CC=C([C@H](C)N(C)C)[C@@]4(C)CC[C@H]23)C1 |t:21| | ||
| Structure | 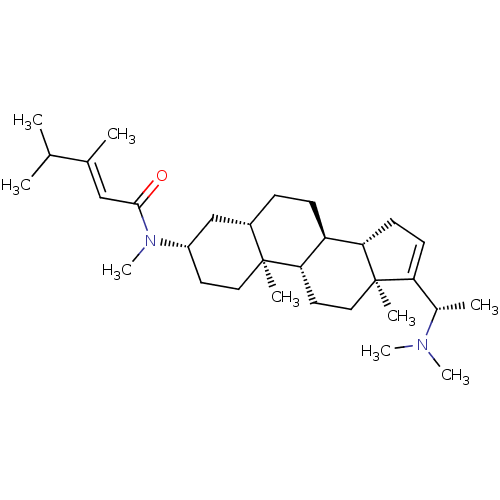
| ||