| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aldo-keto reductase family 1 member C2 |
|---|
| Ligand | BDBM50427664 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_941825 (CHEMBL2329816) |
|---|
| IC50 | 45140±n/a nM |
|---|
| Citation |  Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aldo-keto reductase family 1 member C2 |
|---|
| Name: | Aldo-keto reductase family 1 member C2 |
|---|
| Synonyms: | 3-alpha-HSD3 | 3-alpha-Hydroxysteroid Dehydrogenase Type 3 (AKR1C2) | AK1C2_HUMAN | AKR1C2 | Aldo-keto reductase family 1 member C2 (AK1C2) | Aldo-keto reductase family 1 member C2 (AK1C2a) | Aldo-keto reductase family 1 member C2 (AKR1C2) | Chlordecone reductase homolog HAKRD | DD-2 | DD/BABP | DDH2 | Dihydrodiol dehydrogenase 2 | Dihydrodiol dehydrogenase/bile acid-binding protein | Trans-1,2-dihydrobenzene-1,2-diol dehydrogenase | Type III 3-alpha-hydroxysteroid dehydrogenase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 36739.89 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 323 |
|---|
| Sequence: | MDSKYQCVKLNDGHFMPVLGFGTYAPAEVPKSKALEAVKLAIEAGFHHIDSAHVYNNEEQ
VGLAIRSKIADGSVKREDIFYTSKLWSNSHRPELVRPALERSLKNLQLDYVDLYLIHFPV
SVKPGEEVIPKDENGKILFDTVDLCATWEAMEKCKDAGLAKSIGVSNFNHRLLEMILNKP
GLKYKPVCNQVECHPYFNQRKLLDFCKSKDIVLVAYSALGSHREEPWVDPNSPVLLEDPV
LCALAKKHKRTPALIALRYQLQRGVVVLAKSYNEQRIRQNVQVFEFQLTSEEMKAIDGLN
RNVRYLTLDIFAGPPNYPFSDEY
|
|
|
|---|
| BDBM50427664 |
|---|
| n/a |
|---|
| Name | BDBM50427664 |
|---|
| Synonyms: | CHEMBL2323473 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H19NO5 |
|---|
| Mol. Mass. | 353.3686 |
|---|
| SMILES | COc1ccc(cc1)C(=O)n1c(C)c(CC(O)=O)c2cc(OC)ccc12 |
|---|
| Structure | 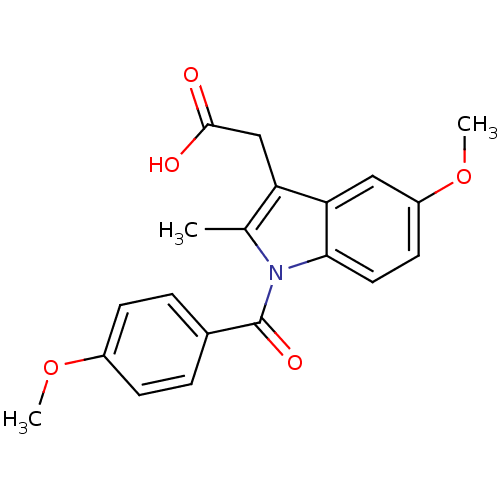
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article
Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article