| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Ephrin type-A receptor 2 |
|---|
| Ligand | BDBM50027856 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_941285 (CHEMBL2329875) |
|---|
| IC50 | 27542±n/a nM |
|---|
| Citation |  Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Ephrin type-A receptor 2 |
|---|
| Name: | Ephrin type-A receptor 2 |
|---|
| Synonyms: | ECK | EPHA2 | EPHA2_HUMAN | Ephrin receptor | Epithelial cell kinase | Tyrosine-protein kinase receptor ECK |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 108260.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1505248 |
|---|
| Residue: | 976 |
|---|
| Sequence: | MELQAARACFALLWGCALAAAAAAQGKEVVLLDFAAAGGELGWLTHPYGKGWDLMQNIMN
DMPIYMYSVCNVMSGDQDNWLRTNWVYRGEAERIFIELKFTVRDCNSFPGGASSCKETFN
LYYAESDLDYGTNFQKRLFTKIDTIAPDEITVSSDFEARHVKLNVEERSVGPLTRKGFYL
AFQDIGACVALLSVRVYYKKCPELLQGLAHFPETIAGSDAPSLATVAGTCVDHAVVPPGG
EEPRMHCAVDGEWLVPIGQCLCQAGYEKVEDACQACSPGFFKFEASESPCLECPEHTLPS
PEGATSCECEEGFFRAPQDPASMPCTRPPSAPHYLTAVGMGAKVELRWTPPQDSGGREDI
VYSVTCEQCWPESGECGPCEASVRYSEPPHGLTRTSVTVSDLEPHMNYTFTVEARNGVSG
LVTSRSFRTASVSINQTEPPKVRLEGRSTTSLSVSWSIPPPQQSRVWKYEVTYRKKGDSN
SYNVRRTEGFSVTLDDLAPDTTYLVQVQALTQEGQGAGSKVHEFQTLSPEGSGNLAVIGG
VAVGVVLLLVLAGVGFFIHRRRKNQRARQSPEDVYFSKSEQLKPLKTYVDPHTYEDPNQA
VLKFTTEIHPSCVTRQKVIGAGEFGEVYKGMLKTSSGKKEVPVAIKTLKAGYTEKQRVDF
LGEAGIMGQFSHHNIIRLEGVISKYKPMMIITEYMENGALDKFLREKDGEFSVLQLVGML
RGIAAGMKYLANMNYVHRDLAARNILVNSNLVCKVSDFGLSRVLEDDPEATYTTSGGKIP
IRWTAPEAISYRKFTSASDVWSFGIVMWEVMTYGERPYWELSNHEVMKAINDGFRLPTPM
DCPSAIYQLMMQCWQQERARRPKFADIVSILDKLIRAPDSLKTLADFDPRVSIRLPSTSG
SEGVPFRTVSEWLESIKMQQYTEHFMAAGYTAIEKVVQMTNDDIKRIGVRLPGHQKRIAY
SLLGLKDQVNTVGIPI
|
|
|
|---|
| BDBM50027856 |
|---|
| n/a |
|---|
| Name | BDBM50027856 |
|---|
| Synonyms: | CHEMBL2323553 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H49NO4S |
|---|
| Mol. Mass. | 507.769 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@H](CCSC)C(O)=O |r| |
|---|
| Structure | 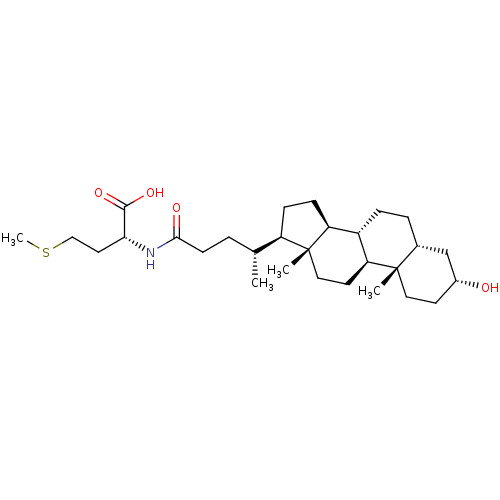
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article
Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article