| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Ephrin type-A receptor 8 |
|---|
| Ligand | BDBM50428059 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_941278 (CHEMBL2329868) |
|---|
| IC50 | 1500±n/a nM |
|---|
| Citation |  Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Ephrin type-A receptor 8 |
|---|
| Name: | Ephrin type-A receptor 8 |
|---|
| Synonyms: | EEK | EPHA8 | EPHA8_HUMAN | Ephrin receptor | Ephrin type-A receptor 8 | HEK3 | KIAA1459 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 111022.09 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_774600 |
|---|
| Residue: | 1005 |
|---|
| Sequence: | MAPARGRLPPALWVVTAAAAAATCVSAARGEVNLLDTSTIHGDWGWLTYPAHGWDSINEV
DESFQPIHTYQVCNVMSPNQNNWLRTSWVPRDGARRVYAEIKFTLRDCNSMPGVLGTCKE
TFNLYYLESDRDLGASTQESQFLKIDTIAADESFTGADLGVRRLKLNTEVRSVGPLSKRG
FYLAFQDIGACLAILSLRIYYKKCPAMVRNLAAFSEAVTGADSSSLVEVRGQCVRHSEER
DTPKMYCSAEGEWLVPIGKCVCSAGYEERRDACVACELGFYKSAPGDQLCARCPPHSHSA
APAAQACHCDLSYYRAALDPPSSACTRPPSAPVNLISSVNGTSVTLEWAPPLDPGGRSDI
TYNAVCRRCPWALSRCEACGSGTRFVPQQTSLVQASLLVANLLAHMNYSFWIEAVNGVSD
LSPEPRRAAVVNITTNQAAPSQVVVIRQERAGQTSVSLLWQEPEQPNGIILEYEIKYYEK
DKEMQSYSTLKAVTTRATVSGLKPGTRYVFQVRARTSAGCGRFSQAMEVETGKPRPRYDT
RTIVWICLTLITGLVVLLLLLICKKRHCGYSKAFQDSDEEKMHYQNGQAPPPVFLPLHHP
PGKLPEPQFYAEPHTYEEPGRAGRSFTREIEASRIHIEKIIGSGDSGEVCYGRLRVPGQR
DVPVAIKALKAGYTERQRRDFLSEASIMGQFDHPNIIRLEGVVTRGRLAMIVTEYMENGS
LDTFLRTHDGQFTIMQLVGMLRGVGAGMRYLSDLGYVHRDLAARNVLVDSNLVCKVSDFG
LSRVLEDDPDAAYTTTGGKIPIRWTAPEAIAFRTFSSASDVWSFGVVMWEVLAYGERPYW
NMTNRDVISSVEEGYRLPAPMGCPHALHQLMLDCWHKDRAQRPRFSQIVSVLDALIRSPE
SLRATATVSRCPPPAFVRSCFDLRGGSGGGGGLTVGDWLDSIRMGRYRDHFAAGGYSSLG
MVLRMNAQDVRALGITLMGHQKKILGSIQTMRAQLTSTQGPRRHL
|
|
|
|---|
| BDBM50428059 |
|---|
| n/a |
|---|
| Name | BDBM50428059 |
|---|
| Synonyms: | CHEMBL2322989 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C35H50N2O4 |
|---|
| Mol. Mass. | 562.7825 |
|---|
| SMILES | C[C@H](CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| |
|---|
| Structure | 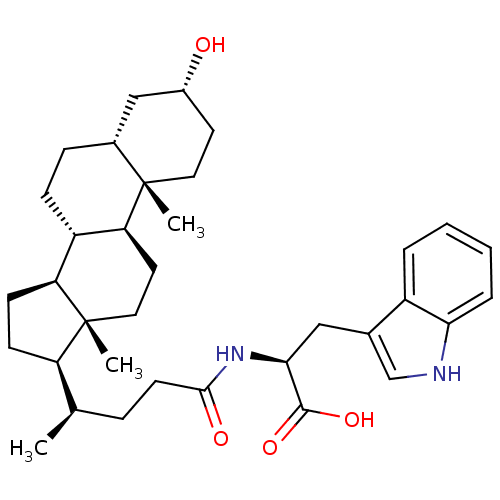
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article
Incerti, M; Tognolini, M; Russo, S; Pala, D; Giorgio, C; Hassan-Mohamed, I; Noberini, R; Pasquale, EB; Vicini, P; Piersanti, S; Rivara, S; Barocelli, E; Mor, M; Lodola, A Amino acid conjugates of lithocholic acid as antagonists of the EphA2 receptor. J Med Chem56:2936-47 (2013) [PubMed] Article